Mouse and human urothelial cancer organoids: A tool for bladder cancer research
- PMID: 30787188
- PMCID: PMC6410883
- DOI: 10.1073/pnas.1803595116
Mouse and human urothelial cancer organoids: A tool for bladder cancer research
Abstract
Bladder cancer is a common malignancy that has a relatively poor outcome. Lack of culture models for the bladder epithelium (urothelium) hampers the development of new therapeutics. Here we present a long-term culture system of the normal mouse urothelium and an efficient culture system of human bladder cancer cells. These so-called bladder (cancer) organoids consist of 3D structures of epithelial cells that recapitulate many aspects of the urothelium. Mouse bladder organoids can be cultured efficiently and genetically manipulated with ease, which was exemplified by creating genetic knockouts in the tumor suppressors Trp53 and Stag2. Human bladder cancer organoids can be derived efficiently from both resected tumors and biopsies and cultured and passaged for prolonged periods. We used this feature of human bladder organoids to create a living biobank consisting of bladder cancer organoids derived from 53 patients. Resulting organoids were characterized histologically and functionally. Organoid lines contained both basal and luminal bladder cancer subtypes based on immunohistochemistry and gene expression analysis. Common bladder cancer mutations like TP53 and FGFR3 were found in organoids in the biobank. Finally, we performed limited drug testing on organoids in the bladder cancer biobank.
Keywords: bladder cancer; organoid; urothelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
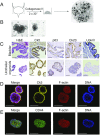

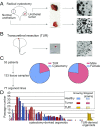
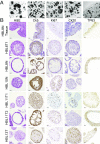
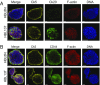
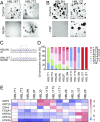
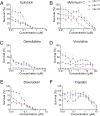
Comment in
-
Re: Mouse and Human Urothelial Cancer Organoids: A Tool for Bladder Cancer Research.J Urol. 2019 Oct;202(4):664. doi: 10.1097/01.JU.0000576984.21354.ad. Epub 2019 Sep 6. J Urol. 2019. PMID: 31287759 No abstract available.
-
Bladder organoids: a step towards personalised cancer therapy?Transl Androl Urol. 2019 Jul;8(Suppl 3):S300-S302. doi: 10.21037/tau.2019.06.10. Transl Androl Urol. 2019. PMID: 31392152 Free PMC article. No abstract available.
-
Establishing and characterization of human and murine bladder cancer organoids.Transl Androl Urol. 2019 Jul;8(Suppl 3):S310-S313. doi: 10.21037/tau.2019.06.05. Transl Androl Urol. 2019. PMID: 31392155 Free PMC article. No abstract available.
Similar articles
-
Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture.Cancer Sci. 2019 Sep;110(9):2806-2821. doi: 10.1111/cas.14118. Epub 2019 Jul 23. Cancer Sci. 2019. PMID: 31254429 Free PMC article.
-
Establishment of 2.5D organoid culture model using 3D bladder cancer organoid culture.Sci Rep. 2020 Jun 10;10(1):9393. doi: 10.1038/s41598-020-66229-w. Sci Rep. 2020. PMID: 32523078 Free PMC article.
-
Organoid model of urothelial cancer: establishment and applications for bladder cancer research.Biotechniques. 2020 Sep;69(3):193-199. doi: 10.2144/btn-2020-0068. Epub 2020 Jul 13. Biotechniques. 2020. PMID: 32654505
-
Urothelial cancer organoids: a tool for bladder cancer research.Pathologe. 2021 Dec;42(Suppl 2):165-169. doi: 10.1007/s00292-021-00988-9. Epub 2021 Oct 8. Pathologe. 2021. PMID: 34623463 Free PMC article. Review.
-
[Organoids as a milestone on the way to personalized treatment of urothelial carcinoma: a systematic review].Urologie. 2022 Jul;61(7):745-752. doi: 10.1007/s00120-022-01854-z. Epub 2022 Jun 13. Urologie. 2022. PMID: 35925247 Review. German.
Cited by
-
Single-Cell Analyses of a Novel Mouse Urothelial Carcinoma Model Reveal a Role of Tumor-Associated Macrophages in Response to Anti-PD-1 Therapy.Cancers (Basel). 2022 May 19;14(10):2511. doi: 10.3390/cancers14102511. Cancers (Basel). 2022. PMID: 35626115 Free PMC article.
-
Tumor organoids: synergistic applications, current challenges, and future prospects in cancer therapy.Cancer Commun (Lond). 2021 Dec;41(12):1331-1353. doi: 10.1002/cac2.12224. Epub 2021 Oct 29. Cancer Commun (Lond). 2021. PMID: 34713636 Free PMC article. Review.
-
Current and innovative approaches in the treatment of non-muscle invasive bladder cancer: the role of transurethral resection of bladder tumor and organoids.Radiol Oncol. 2020 May 2;54(2):135-143. doi: 10.2478/raon-2020-0025. Radiol Oncol. 2020. PMID: 32374292 Free PMC article. Review.
-
Building consensus on the application of organoid-based drug sensitivity testing in cancer precision medicine and drug development.Theranostics. 2024 May 27;14(8):3300-3316. doi: 10.7150/thno.96027. eCollection 2024. Theranostics. 2024. PMID: 38855182 Free PMC article. Review.
-
Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro.Clin Transl Immunology. 2021 Jan 31;10(2):e1248. doi: 10.1002/cti2.1248. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33552510 Free PMC article.
References
-
- Dutch Cancer Registry (NKR) 2018 Available at https://www.cijfersoverkanker.nl/. Accessed February 1, 2018.
-
- Berdik C. Unlocking bladder cancer. Nature. 2017;551:S34–S35. - PubMed
-
- Hermans TJN, et al. Neoadjuvant treatment for muscle-invasive bladder cancer: The past, the present, and the future. Urol Oncol. 2018;36:413–422. - PubMed
-
- Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: Update on tumour genomics and immunotherapy. Nat Rev Urol. 2018;15:92–111. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

