A Phase II Study of Alisertib in Children with Recurrent/Refractory Solid Tumors or Leukemia: Children's Oncology Group Phase I and Pilot Consortium (ADVL0921)
- PMID: 30777875
- PMCID: PMC6897379
- DOI: 10.1158/1078-0432.CCR-18-2675
A Phase II Study of Alisertib in Children with Recurrent/Refractory Solid Tumors or Leukemia: Children's Oncology Group Phase I and Pilot Consortium (ADVL0921)
Abstract
Purpose: Aurora A kinase (AAK) plays an integral role in mitotic entry, DNA damage checkpoint recovery, and centrosome and spindle maturation. Alisertib (MLN8237) is a potent and selective AAK inhibitor. In pediatric preclinical models, antitumor activity was observed in neuroblastoma, acute lymphoblastic leukemia, and sarcoma xenografts. We conducted a phase 2 trial of alisertib in pediatric patients with refractory or recurrent solid tumors or acute leukemias (NCT01154816).
Patients and methods: Alisertib (80 mg/m2/dose) was administered orally, daily for 7 days every 21 days. Pharmacogenomic (PG) evaluation for polymorphisms in the AURK gene and drug metabolizing enzymes (UGT1A1*28), and plasma pharmacokinetic studies (PK) were performed. Using a 2-stage design, patients were enrolled to 12 disease strata (10 solid tumor and 2 acute leukemia). Response was assessed after cycle 1, then every other cycle.
Results: A total of 139 children and adolescents (median age, 10 years) were enrolled, 137 were evaluable for response. Five objective responses were observed (2 complete responses and 3 partial responses). The most frequent toxicity was myelosuppression. The median alisertib trough concentration on day 4 was 1.3 μmol/L, exceeding the 1 μmol/L target trough concentration in 67% of patients. No correlations between PG or PK and toxicity were observed.
Conclusions: Despite alisertib activity in pediatric xenograft models and cogent pharmacokinetic-pharmacodynamic relationships in preclinical models and adults, the objective response rate in children and adolescents receiving single-agent alisertib was less than 5%.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures
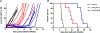
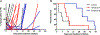
Similar articles
-
Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas.J Clin Oncol. 2014 Jan 1;32(1):44-50. doi: 10.1200/JCO.2012.46.8793. Epub 2013 Sep 16. J Clin Oncol. 2014. PMID: 24043741 Free PMC article. Clinical Trial.
-
Preclinical pharmacokinetic/pharmacodynamic/efficacy relationships for alisertib, an investigational small-molecule inhibitor of Aurora A kinase.Cancer Chemother Pharmacol. 2013 Dec;72(6):1255-64. doi: 10.1007/s00280-013-2305-8. Epub 2013 Oct 8. Cancer Chemother Pharmacol. 2013. PMID: 24101146
-
Population Pharmacokinetics and Exposure-Safety Relationships of Alisertib in Children and Adolescents With Advanced Malignancies.J Clin Pharmacol. 2022 Feb;62(2):206-219. doi: 10.1002/jcph.1958. Epub 2022 Jan 15. J Clin Pharmacol. 2022. PMID: 34435684 Free PMC article. Clinical Trial.
-
Alisertib: a review of pharmacokinetics, efficacy and toxicity in patients with hematologic malignancies and solid tumors.Expert Opin Investig Drugs. 2018 Jan;27(1):105-112. doi: 10.1080/13543784.2018.1417382. Epub 2018 Jan 3. Expert Opin Investig Drugs. 2018. PMID: 29260599 Review.
-
The role of alisertib in treatment of peripheral T-cell lymphomas.Future Oncol. 2015 Sep;11(18):2515-24. doi: 10.2217/fon.15.154. Epub 2015 Sep 7. Future Oncol. 2015. PMID: 26344156 Review.
Cited by
-
Targeted treatment of solid tumors in pediatric precision oncology.Front Oncol. 2023 May 5;13:1176790. doi: 10.3389/fonc.2023.1176790. eCollection 2023. Front Oncol. 2023. PMID: 37213274 Free PMC article. Review.
-
Current Knowledge and Perspectives of Immunotherapies for Neuroblastoma.Cancers (Basel). 2024 Aug 17;16(16):2865. doi: 10.3390/cancers16162865. Cancers (Basel). 2024. PMID: 39199637 Free PMC article. Review.
-
Rhabdoid tumor predisposition syndrome: A historical review of treatments and outcomes for associated pediatric malignancies.Pediatr Blood Cancer. 2024 Jun;71(6):e30979. doi: 10.1002/pbc.30979. Epub 2024 Mar 30. Pediatr Blood Cancer. 2024. PMID: 38553892 Review.
-
Overcoming translational barriers in H3K27-altered diffuse midline glioma: Increasing the drug-tumor residence time.Neurooncol Adv. 2023 Mar 27;5(1):vdad033. doi: 10.1093/noajnl/vdad033. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 37128506 Free PMC article.
-
PROTAC-mediated degradation reveals a non-catalytic function of AURORA-A kinase.Nat Chem Biol. 2020 Nov;16(11):1179-1188. doi: 10.1038/s41589-020-00652-y. Epub 2020 Sep 28. Nat Chem Biol. 2020. PMID: 32989298 Free PMC article.
References
-
- Gautschi O, Heighway J, Mack PC, et al.: Aurora kinases as anticancer drug targets. Clinical cancer research : an official journal of the American Association for Cancer Research 14:1639–48, 2008 - PubMed
-
- Nigg EA: Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2:21–32, 2001 - PubMed
-
- Lapenna S, Giordano A: Cell cycle kinases as therapeutic targets for cancer. Nature reviews. Drug discovery 8:547–66, 2009 - PubMed
-
- Sen S, Zhou H, Zhang RD, et al.: Amplification/overexpression of a mitotic kinase gene in human bladder cancer. Journal of the National Cancer Institute 94:1320–9, 2002 - PubMed
-
- Borges KS, Moreno DA, Martinelli CE Jr., et al.: Spindle assembly checkpoint gene expression in childhood adrenocortical tumors (ACT): Overexpression of Aurora kinases A and B is associated with a poor prognosis. Pediatr Blood Cancer 60:1809–16, 2013 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

