Cryo-EM structures of the DCPIB-inhibited volume-regulated anion channel LRRC8A in lipid nanodiscs
- PMID: 30775971
- PMCID: PMC6395065
- DOI: 10.7554/eLife.42636
Cryo-EM structures of the DCPIB-inhibited volume-regulated anion channel LRRC8A in lipid nanodiscs
Abstract
Hypoosmotic conditions activate volume-regulated anion channels in vertebrate cells. These channels are formed by leucine-rich repeat-containing protein 8 (LRRC8) family members and contain LRRC8A in homo- or hetero-hexameric assemblies. Here, we present single-particle cryo-electron microscopy structures of Mus musculus LRRC8A in complex with the inhibitor DCPIB reconstituted in lipid nanodiscs. DCPIB plugs the channel like a cork in a bottle - binding in the extracellular selectivity filter and sterically occluding ion conduction. Constricted and expanded structures reveal coupled dilation of cytoplasmic LRRs and the channel pore, suggesting a mechanism for channel gating by internal stimuli. Conformational and symmetry differences between LRRC8A structures determined in detergent micelles and lipid bilayers related to reorganization of intersubunit lipid binding sites demonstrate a critical role for the membrane in determining channel structure. These results provide insight into LRRC8 gating and inhibition and the role of lipids in the structure of an ionic-strength sensing ion channel.
Keywords: LRRC8; VRAC; cryo-EM; ion channel; molecular biophysics; mouse; neuroscience; structural biology; volume regulation.
© 2019, Kern et al.
Conflict of interest statement
DK, SO, RH, SB No competing interests declared
Figures





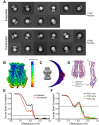

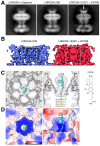
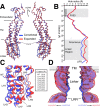






Similar articles
-
Recent Advances in the Structural Biology of the Volume-Regulated Anion Channel LRRC8.Front Pharmacol. 2022 May 11;13:896532. doi: 10.3389/fphar.2022.896532. eCollection 2022. Front Pharmacol. 2022. PMID: 35645818 Free PMC article. Review.
-
LRRC8A homohexameric channels poorly recapitulate VRAC regulation and pharmacology.Am J Physiol Cell Physiol. 2021 Mar 1;320(3):C293-C303. doi: 10.1152/ajpcell.00454.2020. Epub 2020 Dec 23. Am J Physiol Cell Physiol. 2021. PMID: 33356947 Free PMC article.
-
Cryo-EM structures of an LRRC8 chimera with native functional properties reveal heptameric assembly.Elife. 2023 Mar 10;12:e82431. doi: 10.7554/eLife.82431. Elife. 2023. PMID: 36897307 Free PMC article.
-
Structural basis for assembly and lipid-mediated gating of LRRC8A:C volume-regulated anion channels.Nat Struct Mol Biol. 2023 Jun;30(6):841-852. doi: 10.1038/s41594-023-00944-6. Epub 2023 Mar 16. Nat Struct Mol Biol. 2023. PMID: 36928458
-
Mechanisms of Activation of LRRC8 Volume Regulated Anion Channels.Cell Physiol Biochem. 2021 Feb 13;55(S1):41-56. doi: 10.33594/000000329. Cell Physiol Biochem. 2021. PMID: 33577730 Review.
Cited by
-
On the molecular nature of large-pore channels.J Mol Biol. 2021 Aug 20;433(17):166994. doi: 10.1016/j.jmb.2021.166994. Epub 2021 Apr 16. J Mol Biol. 2021. PMID: 33865869 Free PMC article. Review.
-
Recent Advances in the Structural Biology of the Volume-Regulated Anion Channel LRRC8.Front Pharmacol. 2022 May 11;13:896532. doi: 10.3389/fphar.2022.896532. eCollection 2022. Front Pharmacol. 2022. PMID: 35645818 Free PMC article. Review.
-
Properties, Structures, and Physiological Roles of Three Types of Anion Channels Molecularly Identified in the 2010's.Front Physiol. 2021 Dec 23;12:805148. doi: 10.3389/fphys.2021.805148. eCollection 2021. Front Physiol. 2021. PMID: 35002778 Free PMC article. Review.
-
Structures of human pannexin 1 reveal ion pathways and mechanism of gating.Nature. 2020 Aug;584(7822):646-651. doi: 10.1038/s41586-020-2357-y. Epub 2020 Jun 3. Nature. 2020. PMID: 32494015 Free PMC article.
-
Allosteric modulation of LRRC8 channels by targeting their cytoplasmic domains.Nat Commun. 2021 Sep 14;12(1):5435. doi: 10.1038/s41467-021-25742-w. Nat Commun. 2021. PMID: 34521847 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- na/McKnight Endowment Fund for Neuroscience/International
- NYSCF-R-N145/New York Stem Cell Foundation/International
- P41 GM103310/GM/NIGMS NIH HHS/United States
- F32GM128263/GM/NIGMS NIH HHS/United States
- F32 GM128263/GM/NIGMS NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- na/Searle Scholars Program/International
- DP2GM123496-01/GM/NIGMS NIH HHS/United States
- PO CA008748/CA/NCI NIH HHS/United States
- DP2 GM123496/GM/NIGMS NIH HHS/United States
- na/Klingenstein Third Generation Foundation/International
- S10 OD019994/OD/NIH HHS/United States
- na/Robertson Foundation/International
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

