Oocyte-derived E-cadherin acts as a multiple functional factor maintaining the primordial follicle pool in mice
- PMID: 30770786
- PMCID: PMC6377673
- DOI: 10.1038/s41419-018-1208-3
Oocyte-derived E-cadherin acts as a multiple functional factor maintaining the primordial follicle pool in mice
Abstract
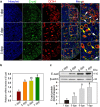
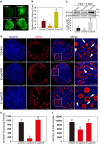
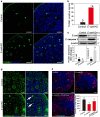
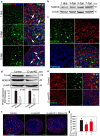
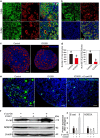
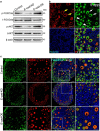
In mammals, female fecundity is determined by the size of the primordial follicle (PF) pool, which is established during the perinatal period. As a non-renewable resource, the preservation of dormant PFs is crucial for sustaining female reproduction throughout life. Although studies have revealed that several oocyte-derived functional genes and pathways, such as newborn ovary homeobox (NOBOX) and 3-phosphoinositide-dependent protein kinase-1, participate in maintaining the PF pool, our understanding of the underlying molecular mechanisms is still incomplete. Here, we demonstrate that E-cadherin (E-cad) plays a crucial role in the maintenance of PFs in mice. E-cad is specifically localized to the cytomembrane of oocytes in PFs. Knockdown of E-cad in neonatal ovaries resulted in significant PF loss owing to oocyte apoptosis. In addition, the expression pattern of NOBOX is similar to that of E-cad. Knockdown of E-cad resulted in a decreased NOBOX level, whereas overexpression of Nobox partially rescued the follicle loss induced by silencing E-cad. Furthermore, E-cad governed NOBOX expression by regulating the shuttle protein, β-catenin, which acts as a transcriptional co-activator. Notably, E-cad, which is a transmembrane protein expressed in the oocytes, was also responsible for maintaining the PF structure by facilitating cell-cell adhesive contacts with surrounding pregranulosa cells. In conclusion, E-cad in oocytes of PFs plays an indispensable role in the maintenance of the PF pool by facilitating follicular structural stability and regulating NOBOX expression. These findings shed light on the physiology of sustaining female reproduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The NOBOX protein becomes undetectable in developmentally competent antral and ovulated oocytes.Int J Dev Biol. 2013;57(1):35-9. doi: 10.1387/ijdb.120125mz. Int J Dev Biol. 2013. PMID: 23585350
-
GDF9 is transiently expressed in oocytes before follicle formation in the human fetal ovary and is regulated by a novel NOBOX transcript.PLoS One. 2015 Mar 19;10(3):e0119819. doi: 10.1371/journal.pone.0119819. eCollection 2015. PLoS One. 2015. PMID: 25790371 Free PMC article.
-
JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice.Development. 2016 May 15;143(10):1778-87. doi: 10.1242/dev.132175. Epub 2016 Mar 24. Development. 2016. PMID: 27013242 Free PMC article.
-
Mechanisms controlling germline cyst breakdown and primordial follicle formation.Cell Mol Life Sci. 2017 Jul;74(14):2547-2566. doi: 10.1007/s00018-017-2480-6. Epub 2017 Feb 14. Cell Mol Life Sci. 2017. PMID: 28197668 Free PMC article. Review.
-
Transcriptional regulation of early oogenesis: in search of masters.Hum Reprod Update. 2006 Jan-Feb;12(1):65-76. doi: 10.1093/humupd/dmi033. Epub 2005 Sep 2. Hum Reprod Update. 2006. PMID: 16143663 Review.
Cited by
-
Mechanisms of primordial follicle activation and new pregnancy opportunity for premature ovarian failure patients.Front Physiol. 2023 Feb 28;14:1113684. doi: 10.3389/fphys.2023.1113684. eCollection 2023. Front Physiol. 2023. PMID: 36926197 Free PMC article. Review.
-
cAMP controls the balance between dormancy and activation of primordial follicles in mouse ovaries.PNAS Nexus. 2023 Feb 21;2(3):pgad055. doi: 10.1093/pnasnexus/pgad055. eCollection 2023 Mar. PNAS Nexus. 2023. PMID: 36938502 Free PMC article.
-
HDAC6 regulates primordial follicle activation through mTOR signaling pathway.Cell Death Dis. 2021 May 29;12(6):559. doi: 10.1038/s41419-021-03842-1. Cell Death Dis. 2021. PMID: 34052832 Free PMC article.
-
The metallic compound promotes primordial follicle activation and ameliorates fertility deficits in aged mice.Theranostics. 2023 May 21;13(10):3131-3148. doi: 10.7150/thno.82553. eCollection 2023. Theranostics. 2023. PMID: 37351158 Free PMC article.
-
The Factors and Pathways Regulating the Activation of Mammalian Primordial Follicles in vivo.Front Cell Dev Biol. 2020 Sep 30;8:575706. doi: 10.3389/fcell.2020.575706. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33102482 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

