Diblock copolymers enhance folding of a mechanosensitive membrane protein during cell-free expression
- PMID: 30760590
- PMCID: PMC6410776
- DOI: 10.1073/pnas.1814775116
Diblock copolymers enhance folding of a mechanosensitive membrane protein during cell-free expression
Abstract
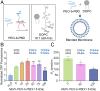
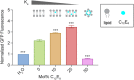
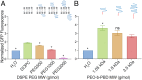
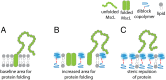
The expression and integration of membrane proteins into vesicle membranes is a critical step in the design of cell-mimetic biosensors, bioreactors, and artificial cells. While membrane proteins have been integrated into a variety of nonnatural membranes, the effects of the chemical and physical properties of these vesicle membranes on protein behavior remain largely unknown. Nonnatural amphiphiles, such as diblock copolymers, provide an interface that can be synthetically controlled to better investigate this relationship. Here, we focus on the initial step in a membrane protein's life cycle: expression and folding. We observe improvements in both the folding and overall production of a model mechanosensitive channel protein, the mechanosensitive channel of large conductance, during cell-free reactions when vesicles containing diblock copolymers are present. By systematically tuning the membrane composition of vesicles through incorporation of a poly(ethylene oxide)-b-poly(butadiene) diblock copolymer, we show that membrane protein folding and production can be improved over that observed in traditional lipid vesicles. We then reproduce this effect with an alternate membrane-elasticizing molecule, C12E8 Our results suggest that global membrane physical properties, specifically available membrane surface area and the membrane area expansion modulus, significantly influence the folding and yield of a membrane protein. Furthermore, our results set the stage for explorations into how nonnatural membrane amphiphiles can be used to both study and enhance the production of biological membrane proteins.
Keywords: cell-free protein synthesis; diblock copolymer; elastic modulus; membrane protein folding; vesicles.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cell-Free Membrane Protein Expression into Hybrid Lipid/Polymer Vesicles.Methods Mol Biol. 2022;2433:257-271. doi: 10.1007/978-1-0716-1998-8_16. Methods Mol Biol. 2022. PMID: 34985750
-
Cell-Free Synthesis of a Transmembrane Mechanosensitive Channel Protein into a Hybrid-Supported Lipid Bilayer.ACS Appl Bio Mater. 2021 Apr 19;4(4):3101-3112. doi: 10.1021/acsabm.0c01482. Epub 2021 Mar 18. ACS Appl Bio Mater. 2021. PMID: 35014398
-
Biocompatibility of poly(epsilon-caprolactone)/poly(ethylene glycol) diblock copolymers with nanophase separation.Biomaterials. 2004 Nov;25(25):5593-601. doi: 10.1016/j.biomaterials.2004.01.061. Biomaterials. 2004. PMID: 15159075
-
Membrane protein synthesis in cell-free systems: from bio-mimetic systems to bio-membranes.FEBS Lett. 2014 Aug 25;588(17):2774-81. doi: 10.1016/j.febslet.2014.06.007. Epub 2014 Jun 12. FEBS Lett. 2014. PMID: 24931371 Review.
-
Emergent properties arising from the assembly of amphiphiles. Artificial vesicle membranes as reaction promoters and regulators.Chem Commun (Camb). 2014 Sep 14;50(71):10177-97. doi: 10.1039/c4cc02812k. Chem Commun (Camb). 2014. PMID: 24921467 Review.
Cited by
-
Enhancing single-cell hyaluronic acid biosynthesis by microbial morphology engineering.Synth Syst Biotechnol. 2020 Sep 30;5(4):316-323. doi: 10.1016/j.synbio.2020.09.002. eCollection 2020 Dec. Synth Syst Biotechnol. 2020. PMID: 33024847 Free PMC article.
-
In Vitro Synthesis and Reconstitution Using Mammalian Cell-Free Lysates Enables the Systematic Study of the Regulation of LINC Complex Assembly.Biochemistry. 2022 Jul 19;61(14):1495-1507. doi: 10.1021/acs.biochem.2c00118. Epub 2022 Jun 23. Biochemistry. 2022. PMID: 35737522 Free PMC article.
-
Engineering transmembrane signal transduction in synthetic membranes using two-component systems.Proc Natl Acad Sci U S A. 2023 May 9;120(19):e2218610120. doi: 10.1073/pnas.2218610120. Epub 2023 May 1. Proc Natl Acad Sci U S A. 2023. PMID: 37126679 Free PMC article.
-
Robust and tunable performance of a cell-free biosensor encapsulated in lipid vesicles.Sci Adv. 2023 Jan 4;9(1):eadd6605. doi: 10.1126/sciadv.add6605. Epub 2023 Jan 4. Sci Adv. 2023. PMID: 36598992 Free PMC article.
-
Unraveling the Phase Behavior, Mechanical Stability, and Protein Reconstitution Properties of Polymer-Lipid Hybrid Vesicles.Biomacromolecules. 2023 Sep 11;24(9):4156-4169. doi: 10.1021/acs.biomac.3c00498. Epub 2023 Aug 4. Biomacromolecules. 2023. PMID: 37539954 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

