Antibody responses to Bordetella pertussis and other childhood vaccines in infants born to mothers who received pertussis vaccine in pregnancy - a prospective, observational cohort study from the United Kingdom
- PMID: 30758857
- PMCID: PMC6591149
- DOI: 10.1111/cei.13275
Antibody responses to Bordetella pertussis and other childhood vaccines in infants born to mothers who received pertussis vaccine in pregnancy - a prospective, observational cohort study from the United Kingdom
Abstract
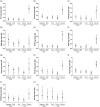
The maternal Tdap (tetanus, diphtheria and acellular pertussis) vaccination programme in the United Kingdom has successfully reduced cases of pertussis in young infants. In addition to prevention of pertussis cases, it is also important to investigate the persistence of maternal antibodies during infancy and the possible interference of maternal antibodies with infant responses to vaccines. We recruited mother-infant pairs from vaccinated and unvaccinated pregnancies and measured concentrations of immunoglobulin (Ig)G against pertussis toxin (PTx), filamentous haemagglutinin (FHA), pertactin (Prn), diphtheria toxin (DTx), tetanus toxoid (TTx) Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae in mothers and infants at birth, and in infants at 7 weeks and at 5 months. Thirty-one mother-infant pairs were tested. Tdap-vaccinated women had significantly higher antibody against Tdap antigens, compared to unvaccinated women (DTx, P = 0·01; PTx, FHA, Prn and TTx, P < 0·001). All antibodies were actively transferred to the infants (transfer ratio > 1) with higher transfer of DTx (P = 0·04) and TTx (P = 0·02) antibody in Tdap-vaccinated pregnancies compared to unvaccinated pregnancies. Infants from Tdap-vaccinated pregnancies had significantly elevated antibodies to all antigens at birth (P < 0.001) and at 7 weeks (FHA, Prn, TTx, P < 0·001; DTx, P = 0.01; PTx, P = 0·004) compared to infants from unvaccinated pregnancies. Infants from Tdap-vaccinated and -unvaccinated pregnancies had comparable antibody concentrations following primary pertussis immunization (PTx, P = 0·77; FHA, P = 0·58; Prn, P = 0·60; DTx, P = 0·09; TTx, P = 0·88). These results support maternal immunization as a method of protecting vulnerable infants during their first weeks of life.
Keywords: antibodies; human; reproductive immunology; vaccination.
© 2019 The Authors. Clinical & Experimental Immunology published by John Wiley & Sons Ltd on behalf of British Society for Immunology.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Antibody responses after primary immunization in infants born to women receiving a pertussis-containing vaccine during pregnancy: single arm observational study with a historical comparator.Clin Infect Dis. 2015 Dec 1;61(11):1637-44. doi: 10.1093/cid/civ695. Epub 2015 Sep 15. Clin Infect Dis. 2015. PMID: 26374816
-
Quantity and Quality of Antibodies After Acellular Versus Whole-cell Pertussis Vaccines in Infants Born to Mothers Who Received Tetanus, Diphtheria, and Acellular Pertussis Vaccine During Pregnancy: A Randomized Trial.Clin Infect Dis. 2020 Jun 24;71(1):72-80. doi: 10.1093/cid/ciz778. Clin Infect Dis. 2020. PMID: 31418814 Clinical Trial.
-
Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial.Lancet Infect Dis. 2019 Apr;19(4):392-401. doi: 10.1016/S1473-3099(18)30717-5. Epub 2019 Mar 27. Lancet Infect Dis. 2019. PMID: 30938299 Clinical Trial.
-
Reduced-antigen, combined diphtheria-tetanus-acellular pertussis vaccine, adsorbed (Boostrix) US formulation): use as a single-dose booster immunization in adolescents aged 10-18 years.Paediatr Drugs. 2006;8(3):189-95; discussion 196. doi: 10.2165/00148581-200608030-00005. Paediatr Drugs. 2006. PMID: 16774298 Review.
-
Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2008 May 30;57(RR-4):1-51. MMWR Recomm Rep. 2008. PMID: 18509304 Review.
Cited by
-
The half-life of maternal transplacental antibodies against diphtheria, tetanus, and pertussis in infants: an individual participant data meta-analysis.Vaccine. 2022 Jan 24;40(3):450-458. doi: 10.1016/j.vaccine.2021.12.007. Epub 2021 Dec 21. Vaccine. 2022. PMID: 34949496 Free PMC article.
-
Quantitative analysis of pertussis, tetanus, and diphtheria antibodies in sera and breast milk from Tdap vaccinated women using a qualified multiplex assay.mSphere. 2024 Apr 23;9(4):e0052723. doi: 10.1128/msphere.00527-23. Epub 2024 Mar 18. mSphere. 2024. PMID: 38497618 Free PMC article.
-
Is the Host Viral Response and the Immunogenicity of Vaccines Altered in Pregnancy?Antibodies (Basel). 2020 Aug 4;9(3):38. doi: 10.3390/antib9030038. Antibodies (Basel). 2020. PMID: 32759839 Free PMC article.
-
Effect of immunization during pregnancy and pre-existing immunity on diphtheria-tetanus-acellular pertussis vaccine responses in infants.Emerg Microbes Infect. 2023 Dec;12(1):2204146. doi: 10.1080/22221751.2023.2204146. Emerg Microbes Infect. 2023. PMID: 37060181 Free PMC article.
-
Antenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization and risk of serogroup 19 IPD in children: An indirect cohort study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2305522. doi: 10.1080/21645515.2024.2305522. Epub 2024 Feb 8. Hum Vaccin Immunother. 2024. PMID: 38330991 Free PMC article.
References
-
- Mills KHG, Ross PJ, Allen AC, Wilk MM. Do we need a new vaccine to control the re‐emergence of pertussis? Trends Microbiol 2014; 22:49–52. - PubMed
-
- Public Health England . Laboratory confirmed cases of pertussis reported to the enhanced pertussis surveillance programme in England: annual report for 2013 ‐ GOV.UK. Available at: https://www.gov.uk/government/publications/pertussis-enhanced-surveillan... (accessed 28 July 2018).
-
- Jones C, Pollock L, Barnett SM, Battersby A, Kampmann B. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine 2014; 32:996–1002. - PubMed

