Association between TDP-43 and mitochondria in inclusion body myositis
- PMID: 30742062
- PMCID: PMC6609472
- DOI: 10.1038/s41374-019-0233-x
Association between TDP-43 and mitochondria in inclusion body myositis
Abstract
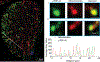
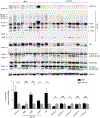
Inclusion body myositis (IBM) is the most common cause of primary myopathy in individuals aged 50 years and over, and is pathologically characterized by protein aggregates of p62 and mislocalized cytoplasmic TDP-43, as well as mitochondrial abnormalities in affected muscle fibers. Our recent studies have shown the accumulation of TDP-43 in mitochondria in neurons from patients with amyotrophic lateral sclerosis (ALS) and frontotemporal degeneration (FTD), and revealed mitochondria as critical mediators of TDP-43 neurotoxicity. In this study, we investigated the association between mitochondria and TDP-43 in biopsied skeletal muscle samples from IBM patients. We found that IBM pathological markers TDP-43, phosphorylated TDP-43, and p62 all coexisted with intensively stained key subunits of mitochondrial oxidative phosphorylation complexes I-V in the same skeletal muscle fibers of patients with IBM. Further immunoblot analysis showed increased levels of TDP-43, truncated TDP-43, phosphorylated TDP-43, and p62, but decreased levels of key subunits of mitochondrial oxidative phosphorylation complexes I and III in IBM patients compared to aged matched control subjects. This is the first demonstration of the close association of TDP-43 accumulation with mitochondria in degenerating muscle fibers in IBM and this association may contribute to the development of mitochondrial dysfunction and pathological protein aggregates.
Conflict of interest statement
Disclosure/Conflict of Interest
The authors declare that there is no conflict of interest.
Figures
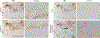
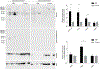
Similar articles
-
Comparative utility of LC3, p62 and TDP-43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathies.Acta Neuropathol Commun. 2013 Jul 1;1:29. doi: 10.1186/2051-5960-1-29. Acta Neuropathol Commun. 2013. PMID: 24252466 Free PMC article.
-
Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis.Muscle Nerve. 2009 Jul;40(1):19-31. doi: 10.1002/mus.21386. Muscle Nerve. 2009. PMID: 19533646 Free PMC article.
-
Phosphorylated TDP-43 aggregates in skeletal and cardiac muscle are a marker of myogenic degeneration in amyotrophic lateral sclerosis and various conditions.Acta Neuropathol Commun. 2019 Oct 28;7(1):165. doi: 10.1186/s40478-019-0824-1. Acta Neuropathol Commun. 2019. PMID: 31661037 Free PMC article.
-
Pathogenic considerations in sporadic inclusion-body myositis, a degenerative muscle disease associated with aging and abnormalities of myoproteostasis.J Neuropathol Exp Neurol. 2012 Aug;71(8):680-93. doi: 10.1097/NEN.0b013e31826183c8. J Neuropathol Exp Neurol. 2012. PMID: 22805774 Review.
-
TDP-43 proteinopathy and mitochondrial abnormalities in neurodegeneration.Mol Cell Neurosci. 2019 Oct;100:103396. doi: 10.1016/j.mcn.2019.103396. Epub 2019 Aug 21. Mol Cell Neurosci. 2019. PMID: 31445085 Free PMC article. Review.
Cited by
-
Inclusion Body Myositis and Neoplasia: A Narrative Review.Int J Mol Sci. 2022 Jul 1;23(13):7358. doi: 10.3390/ijms23137358. Int J Mol Sci. 2022. PMID: 35806366 Free PMC article. Review.
-
Cytoplasmic mislocalization and mitochondrial colocalization of TDP-43 are common features between normal aged and young mice.Exp Biol Med (Maywood). 2020 Nov;245(17):1584-1593. doi: 10.1177/1535370220914253. Epub 2020 Mar 25. Exp Biol Med (Maywood). 2020. PMID: 32212857 Free PMC article.
-
Influence of Inflammatory Cytokines IL-1β and IFNγ on Sarcoplasmic Aggregation of p62 and TDP-43 in Myotubes.Mediators Inflamm. 2023 Sep 12;2023:9018470. doi: 10.1155/2023/9018470. eCollection 2023. Mediators Inflamm. 2023. PMID: 37731843 Free PMC article.
-
Expanding the TDP-43 Proteinopathy Pathway From Neurons to Muscle: Physiological and Pathophysiological Functions.Front Neurosci. 2022 Feb 3;16:815765. doi: 10.3389/fnins.2022.815765. eCollection 2022. Front Neurosci. 2022. PMID: 35185458 Free PMC article. Review.
-
Inclusion body myositis, viral infections, and TDP-43: a narrative review.Clin Exp Med. 2024 May 2;24(1):91. doi: 10.1007/s10238-024-01353-9. Clin Exp Med. 2024. PMID: 38693436 Free PMC article. Review.
References
-
- Callan A, Capkun G, Vasanthaprasad V, et al. A Systematic Review and Meta-Analysis of Prevalence Studies of Sporadic Inclusion Body Myositis. J Neuromuscul Dis 2017;4(2):127–137. - PubMed
-
- Dimachkie MM, Barohn RJ. Inclusion body myositis. Curr Neurol Neurosci Rep 2013;13(1):321. - PubMed
-
- Catalan-Garcia M, Garrabou G, Moren C, et al. Mitochondrial DNA disturbances and deregulated expression of oxidative phosphorylation and mitochondrial fusion proteins in sporadic inclusion body myositis. Clin Sci (Lond) 2016;130(19):1741–1751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

