Identification of Novel Acinetobacter baumannii Host Fatty Acid Stress Adaptation Strategies
- PMID: 30723122
- PMCID: PMC6428749
- DOI: 10.1128/mBio.02056-18
Identification of Novel Acinetobacter baumannii Host Fatty Acid Stress Adaptation Strategies
Abstract
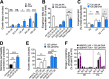
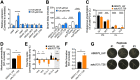
Free fatty acids hold important immune-modulatory roles during infection. However, the host's long-chain polyunsaturated fatty acids, not commonly found in the membranes of bacterial pathogens, also have significant broad-spectrum antibacterial potential. Of these, the omega-6 fatty acid arachidonic acid (AA) and the omega-3 fatty acid decosahexaenoic acid (DHA) are highly abundant; hence, we investigated their effects on the multidrug-resistant human pathogen Acinetobacter baumannii Our analyses reveal that AA and DHA incorporate into the A. baumannii bacterial membrane and impact bacterial fitness and membrane integrity, with DHA having a more pronounced effect. Through transcriptional profiling and mutant analyses, we show that the A. baumannii β-oxidation pathway plays a protective role against AA and DHA, by limiting their incorporation into the phospholipids of the bacterial membrane. Furthermore, our study identified a second bacterial membrane protection system mediated by the AdeIJK efflux system, which modulates the lipid content of the membrane via direct efflux of lipids other than AA and DHA, thereby providing a novel function for this major efflux system in A. baumannii This is the first study to examine the antimicrobial effects of host fatty acids on A. baumannii and highlights the potential of AA and DHA to protect against A. baumannii infections.IMPORTANCE A shift in the Western diet since the industrial revolution has resulted in a dramatic increase in the consumption of omega-6 fatty acids, with a concurrent decrease in the consumption of omega-3 fatty acids. This decrease in omega-3 fatty acid consumption has been associated with significant disease burden, including increased susceptibility to infectious diseases. Here we provide evidence that DHA, an omega-3 fatty acid, has superior antimicrobial effects upon the highly drug-resistant pathogen Acinetobacter baumannii, thereby providing insights into one of the potential health benefits of omega-3 fatty acids. The identification and characterization of two novel bacterial membrane protective mechanisms against host fatty acids provide important insights into A. baumannii adaptation during disease. Furthermore, we describe a novel role for the major multidrug efflux system AdeIJK in A. baumannii membrane maintenance and lipid transport. This core function, beyond drug efflux, increases the appeal of AdeIJK as a therapeutic target.
Keywords: AdeIJK; RND efflux; antimicrobial host lipids; free fatty acids; lipidomics; β-oxidation.
Copyright © 2019 Jiang et al.
Figures
Similar articles
-
The Membrane Composition Defines the Spatial Organization and Function of a Major Acinetobacter baumannii Drug Efflux System.mBio. 2021 Jun 29;12(3):e0107021. doi: 10.1128/mBio.01070-21. Epub 2021 Jun 17. mBio. 2021. PMID: 34134514 Free PMC article.
-
The Impact of Omega-3 Fatty Acids on the Evolution of Acinetobacter baumannii Drug Resistance.Microbiol Spectr. 2021 Dec 22;9(3):e0145521. doi: 10.1128/Spectrum.01455-21. Epub 2021 Nov 17. Microbiol Spectr. 2021. PMID: 34762519 Free PMC article.
-
Contribution of resistance-nodulation-cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumannii.mBio. 2015 Mar 24;6(2):e00309-15. doi: 10.1128/mBio.00309-15. mBio. 2015. PMID: 25805730 Free PMC article.
-
Multidrug resistant Acinetobacter baumannii--the role of AdeABC (RND family) efflux pump in resistance to antibiotics.Folia Histochem Cytobiol. 2008;46(3):257-67. doi: 10.2478/v10042-008-0056-x. Folia Histochem Cytobiol. 2008. PMID: 19056528 Review.
-
[Multi-drug efflux pumps and antibiotic resistance in Acinetobacter baumannii].Rev Chilena Infectol. 2009 Dec;26(6):499-503. Epub 2009 Dec 21. Rev Chilena Infectol. 2009. PMID: 20098782 Review. Spanish.
Cited by
-
To Make or Take: Bacterial Lipid Homeostasis during Infection.mBio. 2021 Jun 29;12(3):e0092821. doi: 10.1128/mBio.00928-21. Epub 2021 Jun 17. mBio. 2021. PMID: 34134515 Free PMC article.
-
Structure, Assembly, and Function of Tripartite Efflux and Type 1 Secretion Systems in Gram-Negative Bacteria.Chem Rev. 2021 May 12;121(9):5479-5596. doi: 10.1021/acs.chemrev.1c00055. Epub 2021 Apr 28. Chem Rev. 2021. PMID: 33909410 Free PMC article. Review.
-
Model architectures for bacterial membranes.Biophys Rev. 2022 Mar 7;14(1):111-143. doi: 10.1007/s12551-021-00913-7. eCollection 2022 Feb. Biophys Rev. 2022. PMID: 35340604 Free PMC article. Review.
-
Molecular Basis of Rhodomyrtone Resistance in Staphylococcus aureus.mBio. 2021 Feb 22;13(1):e0383321. doi: 10.1128/mbio.03833-21. Epub 2022 Feb 15. mBio. 2021. PMID: 35164566 Free PMC article.
-
Metabolomics Reveal Potential Natural Substrates of AcrB in Escherichia coli and Salmonella enterica Serovar Typhimurium.mBio. 2021 Mar 30;12(2):e00109-21. doi: 10.1128/mBio.00109-21. mBio. 2021. PMID: 33785633 Free PMC article.
References
-
- Anes E, Jordao L. 2008. Trick-or-treat: dietary lipids and host resistance to infectious disease. Mini Rev Med Chem 8:1452–1458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
