NEO6860, modality-selective TRPV1 antagonist: a randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain
- PMID: 30706039
- PMCID: PMC6344137
- DOI: 10.1097/PR9.0000000000000696
NEO6860, modality-selective TRPV1 antagonist: a randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain
Abstract
Introduction: NEO6860 is a TRPV1 antagonist when activated by capsaicin but not by heat or pH, developed to relieve pain without the adverse events reported with non-modality-selective TRPV1 antagonists.
Objective: The primary Objective of this study was to evaluate the analgesic efficacy and safety of NEO6860 after 1 day oral dosing in patients with Kellgren-Lawrence stage I, II or III osteoarthritis of the knee.
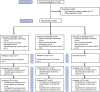
Method: This randomized, double-blinded, 3-period crossover, phase II study compared 1 day (2 doses) of NEO6860 (500 mg twice a day), placebo, and naproxen in 54 patients with osteoarthritis knee pain. Primary endpoint was reduction in pain intensity (PI) on Numerical Rating Scale after exercise, using the staircase test, 8 hours after dose.
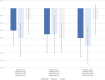
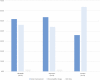
Results: Level of PI, compared with baseline, was numerically lower during NEO6860 and naproxen periods vs placebo at 3 and 24 hours, but not at 8 hours after first dose. A statistically significant effect for naproxen and a trend for NEO6860 were observed at 3 and 24 hours. Least square means' (95% confidence interval) change in PI at 24 hours was -0.67 (-1.09 to -0.26), -0.97 (-1.39 to -0.55), -0.29 (-0.71 to 0.13) for NEO6860, naproxen, and placebo, respectively. NEO6860 exposure was ∼1.6 times higher compared with previous phase I. In this study, NEO6860 safety profile was less favorable than naproxen or placebo. Possibly NEO6860-related adverse events included: feel hot, headache, nausea, dizziness, fatigue, hypoaesthesia, and increased blood pressure.
Conclusion: In this exploratory study, NEO6860 did not statistically significantly outperform placebo but showed an analgesic trend, without impacting body temperature and heat pain perception. Further studies are warranted to explore the potential of NEO6860 in other pain indications. We intent to optimize the dose and evaluate analgesic synergism with other mechanism.
Keywords: Analgesic effect; Change in heat pain perception; Hyperthermia; NEO6860; Osteoarthritis of the knee; Staircase test; TRPV1 antagonist.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
Similar articles
-
Safety, Pharmacokinetics, and Pharmacodynamics Study in Healthy Subjects of Oral NEO6860, a Modality Selective Transient Receptor Potential Vanilloid Subtype 1 Antagonist.J Pain. 2017 Jun;18(6):726-738. doi: 10.1016/j.jpain.2017.01.009. Epub 2017 Feb 8. J Pain. 2017. PMID: 28188907 Clinical Trial.
-
A randomized study to evaluate the analgesic efficacy of a single dose of the TRPV1 antagonist mavatrep in patients with osteoarthritis.Scand J Pain. 2017 Oct;17:134-143. doi: 10.1016/j.sjpain.2017.07.021. Epub 2017 Aug 24. Scand J Pain. 2017. PMID: 28850367 Clinical Trial.
-
A 2-week, multicenter, randomized, double-blind, placebo-controlled, dose-ranging, phase III trial comparing the efficacy of oxymorphone extended release and placebo in adults with pain associated with osteoarthritis of the hip or knee.Clin Ther. 2006 Mar;28(3):352-64. doi: 10.1016/j.clinthera.2006.03.008. Clin Ther. 2006. PMID: 16750450 Clinical Trial.
-
Nitronaproxen: AZD 3582, HCT 3012, Naproxen Nitroxybutylester, NO-Naproxen.Drugs R D. 2006;7(4):262-6. doi: 10.2165/00126839-200607040-00007. Drugs R D. 2006. PMID: 16784252 Review.
-
Chondroitin for osteoarthritis.Cochrane Database Syst Rev. 2015 Jan 28;1(1):CD005614. doi: 10.1002/14651858.CD005614.pub2. Cochrane Database Syst Rev. 2015. PMID: 25629804 Free PMC article. Review.
Cited by
-
Small molecule inhibitors of osteoarthritis: Current development and future perspective.Front Physiol. 2023 Apr 7;14:1156913. doi: 10.3389/fphys.2023.1156913. eCollection 2023. Front Physiol. 2023. PMID: 37089415 Free PMC article. Review.
-
An update on targets for treating osteoarthritis pain: NGF and TRPV1.Curr Treatm Opt Rheumatol. 2020 Sep;6(3):129-145. doi: 10.1007/s40674-020-00146-x. Epub 2020 May 6. Curr Treatm Opt Rheumatol. 2020. PMID: 34178580 Free PMC article.
-
Challenges and opportunities of pharmacological interventions for osteoarthritis: A review of current clinical trials and developments.Osteoarthr Cartil Open. 2021 Sep 8;3(4):100212. doi: 10.1016/j.ocarto.2021.100212. eCollection 2021 Dec. Osteoarthr Cartil Open. 2021. PMID: 36474768 Free PMC article. Review.
-
Antioxidative and Analgesic Effects of Naringin through Selective Inhibition of Transient Receptor Potential Vanilloid Member 1.Antioxidants (Basel). 2021 Dec 28;11(1):64. doi: 10.3390/antiox11010064. Antioxidants (Basel). 2021. PMID: 35052566 Free PMC article.
-
The Mysteries of Capsaicin-Sensitive Afferents.Front Physiol. 2020 Dec 16;11:554195. doi: 10.3389/fphys.2020.554195. eCollection 2020. Front Physiol. 2020. PMID: 33391007 Free PMC article. Review.
References
-
- Available at: https://www.cdc.gov/drugoverdose/data/analysis.html. Accessed May 15, 2018.
-
- Available at: http://www.womac.org/womac/index.htm. Accessed May 15, 2018.
-
- Aiyejusunle C, Kola-Korolo T, Ajiboye O. Comparison of the effects of tens and sodium salicylate iontophoresis in the management of osteoarthritis of the knee. Nig Q J Hosp Med 2007;17:30–4. - PubMed
-
- Bannuru R, Schmid C, Kent D, Vaysbrot E, Wong J, McAlindon T. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015;162:46–54. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
