Development and organization of the zebrafish intestinal epithelial stem cell niche
- PMID: 30698914
- PMCID: PMC8432741
- DOI: 10.1002/dvdy.16
Development and organization of the zebrafish intestinal epithelial stem cell niche
Abstract
Background: Development of the vertebrate intestinal epithelial stem cell niche begins during embryogenesis but maturation occurs postembryonic. The intestinal mammalian crypt contains stem cells interspersed by secretory cells that play a role in regulation of proliferation. Epithelial cells are specified as either secretory or enterocytes as they migrate up the villi in mammals or fold in zebrafish. Zebrafish forms a functional intestine by the end of embryogenesis but takes another 4 weeks to develop the adult proliferation pattern.
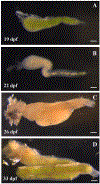
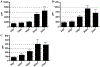
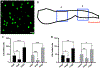
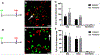
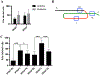
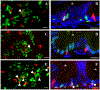
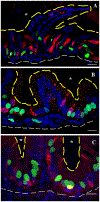
Results: We characterize development of the intestinal epithelial stem cell niche during the postembryonic period. During the first 2-weeks postembryogenesis, different groups of epithelial cells sequentially proceed through one or two cell cycles, appear to become quiescent, and remain at the interfold base. The third week begins asymmetric divisions with proliferative progeny moving up the folds. Apoptotic cells are not observed at the fold tip until the end of the fourth week. Secretory cells intersperse among interfold base proliferative cells, increasing in number during the third and fourth weeks with a coincident change in proliferation pattern.
Conclusions: Zebrafish postembryonic intestinal epithelial development consists of 2 weeks of slow proliferation followed by 2 weeks of metamorphosis to the adult structure. Developmental Dynamics 2019. © 2019 Wiley Periodicals, Inc.
Keywords: development; epithelial; intestine; post-embryonic; proliferation; secretory cell; zebrafish.
© 2019 Wiley Periodicals, Inc.
Figures

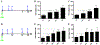



Similar articles
-
A novel group of secretory cells regulates development of the immature intestinal stem cell niche through repression of the main signaling pathways driving proliferation.Dev Biol. 2019 Dec 1;456(1):47-62. doi: 10.1016/j.ydbio.2019.08.005. Epub 2019 Aug 6. Dev Biol. 2019. PMID: 31398318 Free PMC article.
-
Evolution of pig intestinal stem cells from birth to weaning.Animal. 2019 Dec;13(12):2830-2839. doi: 10.1017/S1751731119001319. Epub 2019 Jun 14. Animal. 2019. PMID: 31199215
-
Loss of ascl1a prevents secretory cell differentiation within the zebrafish intestinal epithelium resulting in a loss of distal intestinal motility.Dev Biol. 2013 Apr 15;376(2):171-86. doi: 10.1016/j.ydbio.2013.01.013. Epub 2013 Jan 23. Dev Biol. 2013. PMID: 23353550 Free PMC article.
-
Establishment of intestinal stem cell niche during amphibian metamorphosis.Curr Top Dev Biol. 2013;103:305-27. doi: 10.1016/B978-0-12-385979-2.00011-3. Curr Top Dev Biol. 2013. PMID: 23347524 Review.
-
Microenvironmental regulation of intestinal stem cells in the inflamed intestine.Life Sci. 2021 May 15;273:119298. doi: 10.1016/j.lfs.2021.119298. Epub 2021 Mar 2. Life Sci. 2021. PMID: 33667519 Review.
Cited by
-
The E. coli transcription factor GrlA is regulated by subcellular compartmentalization and activated in response to mechanical stimuli.Proc Natl Acad Sci U S A. 2020 Apr 28;117(17):9519-9528. doi: 10.1073/pnas.1917500117. Epub 2020 Apr 10. Proc Natl Acad Sci U S A. 2020. PMID: 32277032 Free PMC article.
-
Single-cell analysis of shared signatures and transcriptional diversity during zebrafish development.Dev Cell. 2023 Dec 18;58(24):3028-3047.e12. doi: 10.1016/j.devcel.2023.11.001. Epub 2023 Nov 22. Dev Cell. 2023. PMID: 37995681 Free PMC article.
-
Dietary Microplastic Administration during Zebrafish (Danio rerio) Development: A Comprehensive and Comparative Study between Larval and Juvenile Stages.Animals (Basel). 2023 Jul 10;13(14):2256. doi: 10.3390/ani13142256. Animals (Basel). 2023. PMID: 37508033 Free PMC article.
-
Cell clusters containing intestinal stem cells line, the zebrafish intestine intervillus pocket.iScience. 2022 Apr 22;25(5):104280. doi: 10.1016/j.isci.2022.104280. eCollection 2022 May 20. iScience. 2022. PMID: 35586068 Free PMC article.
-
The 3D Pattern of the Rainbow Trout (Oncorhynchus mykiss) Enterocytes and Intestinal Stem Cells.Int J Mol Sci. 2020 Dec 2;21(23):9192. doi: 10.3390/ijms21239192. Int J Mol Sci. 2020. PMID: 33276531 Free PMC article.
References
-
- Al-Nafussi AI, Wright NA. 1982. Cell kinetics in the mouse small intestine during immediate postnatal life. Virchows Arch B Cell Pathol Incl Mol Pathol 40:51–62. - PubMed
-
- Barker N 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15:19–33. - PubMed
-
- Bjerknes M, Cheng H. 1999. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116:7–14. - PubMed
-
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J. 2005. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132:1093–1104. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

