Cytomegalovirus vectors expressing Plasmodium knowlesi antigens induce immune responses that delay parasitemia upon sporozoite challenge
- PMID: 30673723
- PMCID: PMC6343944
- DOI: 10.1371/journal.pone.0210252
Cytomegalovirus vectors expressing Plasmodium knowlesi antigens induce immune responses that delay parasitemia upon sporozoite challenge
Abstract
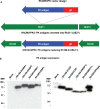
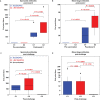
The development of a sterilizing vaccine against malaria remains one of the highest priorities for global health research. While sporozoite vaccines targeting the pre-erythrocytic stage show great promise, it has not been possible to maintain efficacy long-term, likely due to an inability of these vaccines to maintain effector memory T cell responses in the liver. Vaccines based on human cytomegalovirus (HCMV) might overcome this limitation since vectors based on rhesus CMV (RhCMV), the homologous virus in rhesus macaques (RM), elicit and indefinitely maintain high frequency, non-exhausted effector memory T cells in extralymphoid tissues, including the liver. Moreover, RhCMV strain 68-1 elicits CD8+ T cells broadly recognizing unconventional epitopes exclusively restricted by MHC-II and MHC-E. To evaluate the potential of these unique immune responses to protect against malaria, we expressed four Plasmodium knowlesi (Pk) antigens (CSP, AMA1, SSP2/TRAP, MSP1c) in RhCMV 68-1 or in Rh189-deleted 68-1, which additionally elicits canonical MHC-Ia-restricted CD8+ T cells. Upon inoculation of RM with either of these Pk Ag expressing RhCMV vaccines, we obtained T cell responses to each of the four Pk antigens. Upon challenge with Pk sporozoites we observed a delayed appearance of blood stage parasites in vaccinated RM consistent with a 75-80% reduction of parasite release from the liver. Moreover, the Rh189-deleted RhCMV/Pk vectors elicited sterile protection in one RM. Once in the blood, parasite growth was not affected. In contrast to T cell responses induced by Pk infection, RhCMV vectors maintained sustained T cell responses to all four malaria antigens in the liver post-challenge. The delayed appearance of blood stage parasites is thus likely due to a T cell-mediated inhibition of liver stage parasite development. As such, this vaccine approach can be used to efficiently test new T cell antigens, improve current vaccines targeting the liver stage and complement vaccines targeting erythrocytic antigens.
Conflict of interest statement
OHSU and Drs. Picker, Hansen, and Früh have a significant financial interest in VIR Biotechnology Inc., a company that may have a commercial interest in the results of this research and technology. The potential individual and institutional conflicts of interest have been reviewed and managed by OHSU. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Malaria infection by sporozoite challenge induces high functional antibody titres against blood stage antigens after a DNA prime, poxvirus boost vaccination strategy in Rhesus macaques.Malar J. 2011 Feb 8;10:29. doi: 10.1186/1475-2875-10-29. Malar J. 2011. PMID: 21303498 Free PMC article.
-
Sterile protection against Plasmodium knowlesi in rhesus monkeys from a malaria vaccine: comparison of heterologous prime boost strategies.PLoS One. 2009 Aug 10;4(8):e6559. doi: 10.1371/journal.pone.0006559. PLoS One. 2009. PMID: 19668343 Free PMC article.
-
Chemoprophylaxis with sporozoite immunization in P. knowlesi rhesus monkeys confers protection and elicits sporozoite-specific memory T cells in the liver.PLoS One. 2017 Feb 9;12(2):e0171826. doi: 10.1371/journal.pone.0171826. eCollection 2017. PLoS One. 2017. PMID: 28182750 Free PMC article.
-
Memory T cells maintain protracted protection against malaria.Immunol Lett. 2014 Oct;161(2):189-95. doi: 10.1016/j.imlet.2014.03.011. Epub 2014 Apr 5. Immunol Lett. 2014. PMID: 24709142 Free PMC article. Review.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
Cited by
-
Construction and Characterization of a High-Capacity Replication-Competent Murine Cytomegalovirus Vector for Gene Delivery.Vaccines (Basel). 2024 Jul 18;12(7):791. doi: 10.3390/vaccines12070791. Vaccines (Basel). 2024. PMID: 39066429 Free PMC article.
-
Late gene expression-deficient cytomegalovirus vectors elicit conventional T cells that do not protect against SIV.JCI Insight. 2023 Mar 22;8(6):e164692. doi: 10.1172/jci.insight.164692. JCI Insight. 2023. PMID: 36749635 Free PMC article.
-
Vaccination With Sporozoites: Models and Correlates of Protection.Front Immunol. 2019 Jun 5;10:1227. doi: 10.3389/fimmu.2019.01227. eCollection 2019. Front Immunol. 2019. PMID: 31231377 Free PMC article. Review.
-
Cell-Mediated Immunity Generated in Response to a Purified Inactivated Vaccine for Dengue Virus Type 1.mSphere. 2020 Jan 22;5(1):e00671-19. doi: 10.1128/mSphere.00671-19. mSphere. 2020. PMID: 31969476 Free PMC article. Clinical Trial.
-
Novel Strategies to Combat CMV-Related Cardiovascular Disease.Pathog Immun. 2020 Sep 20;5(1):240-274. doi: 10.20411/pai.v5i1.382. eCollection 2020. Pathog Immun. 2020. PMID: 33089035 Free PMC article. Review.
References
-
- WHO. World Malaria Report 2014. 2014; Available from: http://www.who.int/malaria/publications/world_malaria_report_2014/report/en.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

