HIF-1α metabolically controls collagen synthesis and modification in chondrocytes
- PMID: 30651640
- PMCID: PMC7195049
- DOI: 10.1038/s41586-019-0874-3
HIF-1α metabolically controls collagen synthesis and modification in chondrocytes
Abstract
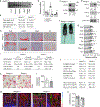
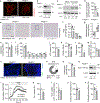
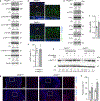
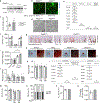
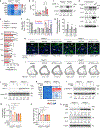
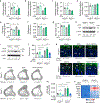
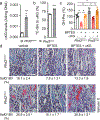
Endochondral ossification, an important process in vertebrate bone formation, is highly dependent on correct functioning of growth plate chondrocytes1. Proliferation of these cells determines longitudinal bone growth and the matrix deposited provides a scaffold for future bone formation. However, these two energy-dependent anabolic processes occur in an avascular environment1,2. In addition, the centre of the expanding growth plate becomes hypoxic, and local activation of the hypoxia-inducible transcription factor HIF-1α is necessary for chondrocyte survival by unidentified cell-intrinsic mechanisms3-6. It is unknown whether there is a requirement for restriction of HIF-1α signalling in the other regions of the growth plate and whether chondrocyte metabolism controls cell function. Here we show that prolonged HIF-1α signalling in chondrocytes leads to skeletal dysplasia by interfering with cellular bioenergetics and biosynthesis. Decreased glucose oxidation results in an energy deficit, which limits proliferation, activates the unfolded protein response and reduces collagen synthesis. However, enhanced glutamine flux increases α-ketoglutarate levels, which in turn increases proline and lysine hydroxylation on collagen. This metabolically regulated collagen modification renders the cartilaginous matrix more resistant to protease-mediated degradation and thereby increases bone mass. Thus, inappropriate HIF-1α signalling results in skeletal dysplasia caused by collagen overmodification, an effect that may also contribute to other diseases involving the extracellular matrix such as cancer and fibrosis.
Figures




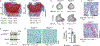

Similar articles
-
Hypoxia-inducible factor-1 (HIF-1) but not HIF-2 is essential for hypoxic induction of collagen prolyl 4-hydroxylases in primary newborn mouse epiphyseal growth plate chondrocytes.J Biol Chem. 2012 Oct 26;287(44):37134-44. doi: 10.1074/jbc.M112.352872. Epub 2012 Aug 28. J Biol Chem. 2012. PMID: 22930750 Free PMC article.
-
Conditional Deletion of Prolyl Hydroxylase Domain-Containing Protein 2 (Phd2) Gene Reveals Its Essential Role in Chondrocyte Function and Endochondral Bone Formation.Endocrinology. 2016 Jan;157(1):127-40. doi: 10.1210/en.2015-1473. Epub 2015 Nov 12. Endocrinology. 2016. PMID: 26562260 Free PMC article.
-
HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes.J Cell Sci. 2003 May 1;116(Pt 9):1819-26. doi: 10.1242/jcs.00385. J Cell Sci. 2003. PMID: 12665562
-
Hypoxia and osteoarthritis: how chondrocytes survive hypoxic environments.Curr Opin Rheumatol. 2007 Sep;19(5):457-62. doi: 10.1097/BOR.0b013e3282ba5693. Curr Opin Rheumatol. 2007. PMID: 17762611 Review.
-
Posttranslational modifications of collagens as targets of hypoxia and Hif-1alpha in endochondral bone development.Ann N Y Acad Sci. 2010 Mar;1192:317-21. doi: 10.1111/j.1749-6632.2009.05236.x. Ann N Y Acad Sci. 2010. PMID: 20392253 Free PMC article. Review.
Cited by
-
A critical bioenergetic switch is regulated by IGF2 during murine cartilage development.Commun Biol. 2022 Nov 11;5(1):1230. doi: 10.1038/s42003-022-04156-4. Commun Biol. 2022. PMID: 36369360 Free PMC article.
-
Electroacupuncture improves articular microcirculation and attenuates cartilage hypoxia in a male rabbit model of knee osteoarthritis.J Tradit Complement Med. 2024 Jan 6;14(4):414-423. doi: 10.1016/j.jtcme.2024.01.002. eCollection 2024 Jul. J Tradit Complement Med. 2024. PMID: 39035691 Free PMC article.
-
Metabolomic Phenotypes Reflect Patient Sex and Injury Status: A Cross-Sectional Analysis of Human Synovial Fluid.bioRxiv [Preprint]. 2023 Feb 4:2023.02.03.527040. doi: 10.1101/2023.02.03.527040. bioRxiv. 2023. Update in: Osteoarthritis Cartilage. 2024 Sep;32(9):1074-1083. doi: 10.1016/j.joca.2023.09.004 PMID: 36846378 Free PMC article. Updated. Preprint.
-
Breast cancer cells rely on environmental pyruvate to shape the metastatic niche.Nature. 2019 Apr;568(7750):117-121. doi: 10.1038/s41586-019-0977-x. Epub 2019 Feb 27. Nature. 2019. PMID: 30814728 Free PMC article.
-
HIF-1α Affects the Neural Stem Cell Differentiation of Human Induced Pluripotent Stem Cells via MFN2-Mediated Wnt/β-Catenin Signaling.Front Cell Dev Biol. 2021 Jun 21;9:671704. doi: 10.3389/fcell.2021.671704. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34235146 Free PMC article.
References
Main text references
-
- Pfander D, Cramer T, Schipani E & Johnson RS HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci 116, 1819–1826 (2003). - PubMed
Methods references
-
- Ovchinnikov DA, Deng JM, Ogunrinu G & Behringer RR Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26, 145–146 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

