Molecular Mechanism of Cytokinesis
- PMID: 30649923
- PMCID: PMC6588489
- DOI: 10.1146/annurev-biochem-062917-012530
Molecular Mechanism of Cytokinesis
Abstract
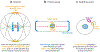
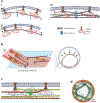
Division of amoebas, fungi, and animal cells into two daughter cells at the end of the cell cycle depends on a common set of ancient proteins, principally actin filaments and myosin-II motors. Anillin, formins, IQGAPs, and many other proteins regulate the assembly of the actin filaments into a contractile ring positioned between the daughter nuclei by different mechanisms in fungi and animal cells. Interactions of myosin-II with actin filaments produce force to assemble and then constrict the contractile ring to form a cleavage furrow. Contractile rings disassemble as they constrict. In some cases, knowledge about the numbers of participating proteins and their biochemical mechanisms has made it possible to formulate molecularly explicit mathematical models that reproduce the observed physical events during cytokinesis by computer simulations.
Keywords: actin; contractile ring; cytokinesis; mathematical models; myosin.
Figures
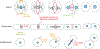
Similar articles
-
Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis.Curr Biol. 2008 Jan 8;18(1):30-6. doi: 10.1016/j.cub.2007.11.068. Epub 2007 Dec 27. Curr Biol. 2008. PMID: 18158243
-
Anillin propels myosin-independent constriction of actin rings.Nat Commun. 2021 Jul 28;12(1):4595. doi: 10.1038/s41467-021-24474-1. Nat Commun. 2021. PMID: 34321459 Free PMC article.
-
Three myosins contribute uniquely to the assembly and constriction of the fission yeast cytokinetic contractile ring.Curr Biol. 2015 Aug 3;25(15):1955-65. doi: 10.1016/j.cub.2015.06.018. Epub 2015 Jul 2. Curr Biol. 2015. PMID: 26144970 Free PMC article.
-
Regulation of cytokinesis.Cell Mol Life Sci. 1999 Jan;55(1):108-20. doi: 10.1007/s000180050274. Cell Mol Life Sci. 1999. PMID: 10065156 Free PMC article. Review.
-
Myosins in Cytokinesis.Adv Exp Med Biol. 2020;1239:233-244. doi: 10.1007/978-3-030-38062-5_11. Adv Exp Med Biol. 2020. PMID: 32451862 Review.
Cited by
-
The Unusual Suspects in Cytokinesis: Fitting the Pieces Together.Front Cell Dev Biol. 2020 Jun 18;8:441. doi: 10.3389/fcell.2020.00441. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32626704 Free PMC article. Review.
-
Profilin choreographs actin and microtubules in cells and cancer.Int Rev Cell Mol Biol. 2020;355:155-204. doi: 10.1016/bs.ircmb.2020.05.005. Epub 2020 Jul 16. Int Rev Cell Mol Biol. 2020. PMID: 32859370 Free PMC article.
-
Physical mechanisms of platelet formation.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):21841-21843. doi: 10.1073/pnas.2014390117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788351 Free PMC article. No abstract available.
-
Actin assembly requirements of the formin Fus1 to build the fusion focus.J Cell Sci. 2022 Jul 1;135(13):jcs260289. doi: 10.1242/jcs.260289. Epub 2022 Jul 8. J Cell Sci. 2022. PMID: 35673994 Free PMC article.
-
Wrangling Actin Assemblies: Actin Ring Dynamics during Cell Wound Repair.Cells. 2022 Sep 6;11(18):2777. doi: 10.3390/cells11182777. Cells. 2022. PMID: 36139352 Free PMC article. Review.
References
-
- Schroeder TE. 1970. The contractile ring. I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z. Zellforsch. Mikrosk. Anat 109:431–49 - PubMed
-
- Rappaport R 1967. Cell division: direct measurement of maximum tension exerted by furrow of echinoderm eggs. Science 156:1241–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

