Co-delivery doxorubicin and silybin for anti-hepatoma via enhanced oral hepatic-targeted efficiency
- PMID: 30643408
- PMCID: PMC6314320
- DOI: 10.2147/IJN.S187888
Co-delivery doxorubicin and silybin for anti-hepatoma via enhanced oral hepatic-targeted efficiency
Abstract
Background: To establish the combination of doxorubicin (DOX) and silybin (SLB) in oral hepatic-targeting liposomes with the goal of reducing cardiotoxic side effects and improve oral hepatoma treatment.
Methods: Distearoylphosphatidylethanolamine-polyethylene glycol-cholic acid-modified liposomes (CA-LP) were used to encapsulate DOX and SLB (CA-LP-DOX/SLB), and the hepatic targeting, efficacy against hepatoma and cardioprotective effects were evaluated by cell toxicity, scratch and apoptosis in vitro studies, and pharmacokinetics and pharmacodynamics in vivo studies.
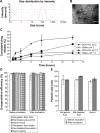
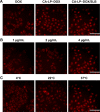
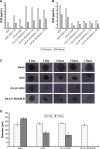
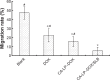
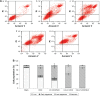
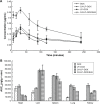
Results: In vitro cell studies showed that CA-LP-DOX/SLB inhibited HepG2 cell proliferation and HCC97H cell migration, and protected H9c2 cells. In vivo pharmacokinetics demonstrated that the CA-LP-DOX/SLB-treated group showed higher liver accumulation and lower heart accumulation of DOX relative to those in the CA-LP-DOX and LP-DOX-treated groups. In vivo pharmacodynamic studies showed that the CA-LP-DOX/SLB-treated group not only efficiently inhibited growth but also induced significantly less tissue damage than that observed in the CA-LP-DOX-treated group.
Conclusion: Concurrent administration of DOX and SLB via CA-LP provided a viable strategy to mitigate acute DOX-induced cardiotoxicity.
Keywords: anti-hepatoma; biodistribution in vivo; cholic acid transporter; doxorubicin; hepatic targeting via oral administration; silybin.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Enhanced doxorubicin delivery to hepatocellular carcinoma cells via CD147 antibody-conjugated immunoliposomes.Nanomedicine. 2018 Aug;14(6):1949-1961. doi: 10.1016/j.nano.2017.09.012. Epub 2017 Oct 16. Nanomedicine. 2018. PMID: 29045824
-
Glycyrrhetinic acid-conjugated polymeric prodrug micelles co-delivered with doxorubicin as combination therapy treatment for liver cancer.Colloids Surf B Biointerfaces. 2019 Mar 1;175:106-115. doi: 10.1016/j.colsurfb.2018.11.082. Epub 2018 Nov 30. Colloids Surf B Biointerfaces. 2019. PMID: 30529816
-
Dual-Ligand-Functionalized Liposomes Based on Glycyrrhetinic Acid and cRGD for Hepatocellular Carcinoma Targeting and Therapy.Mol Pharm. 2023 Apr 3;20(4):1951-1963. doi: 10.1021/acs.molpharmaceut.2c00842. Epub 2023 Mar 23. Mol Pharm. 2023. PMID: 36952242
-
In vivo pharmacokinetics, biodistribution and the anti-tumor effect of cyclic RGD-modified doxorubicin-loaded polymers in tumor-bearing mice.Colloids Surf B Biointerfaces. 2016 Oct 1;146:31-8. doi: 10.1016/j.colsurfb.2016.05.054. Epub 2016 May 18. Colloids Surf B Biointerfaces. 2016. PMID: 27244048
-
Lactosylated liposomes for targeted delivery of doxorubicin to hepatocellular carcinoma.Int J Nanomedicine. 2012;7:5465-74. doi: 10.2147/IJN.S33965. Epub 2012 Oct 16. Int J Nanomedicine. 2012. PMID: 23093902 Free PMC article.
Cited by
-
Preparation, Characterization, and Antioxidant Properties of Phycocyanin Complexes Based on Sodium Alginate and Lysozyme.Front Nutr. 2022 May 24;9:890942. doi: 10.3389/fnut.2022.890942. eCollection 2022. Front Nutr. 2022. PMID: 35685875 Free PMC article.
-
Exploiting gender-based biomarkers and drug targets: advancing personalized therapeutic strategies in hepatocellular carcinoma.Front Pharmacol. 2024 Jun 20;15:1433540. doi: 10.3389/fphar.2024.1433540. eCollection 2024. Front Pharmacol. 2024. PMID: 38966543 Free PMC article. Review.
-
Recent advances of novel targeted drug delivery systems based on natural medicine monomers against hepatocellular carcinoma.Heliyon. 2024 Jan 18;10(2):e24667. doi: 10.1016/j.heliyon.2024.e24667. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38312669 Free PMC article. Review.
-
Naringenin Regulates Doxorubicin-Induced Liver Dysfunction: Impact on Oxidative Stress and Inflammation.Plants (Basel). 2020 Apr 24;9(4):550. doi: 10.3390/plants9040550. Plants (Basel). 2020. PMID: 32344607 Free PMC article.
-
The feasibility of oral targeted drug delivery: Gut immune to particulates?Acta Pharm Sin B. 2023 Jun;13(6):2544-2558. doi: 10.1016/j.apsb.2022.10.020. Epub 2022 Oct 29. Acta Pharm Sin B. 2023. PMID: 37425061 Free PMC article. Review.
References
-
- Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10(1):34–42. - PubMed
-
- Zhang X, Ng HLH, Lu A, et al. Drug delivery system targeting advanced hepatocellular carcinoma: current and future. Nanomedicine. 2016;12(4):853–869. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Dong Y, Feng SS, Poly FSS. Poly(d,l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(30):6068–6076. - PubMed
-
- Sun JB, Duan JH, Dai SL, et al. In vitro and in vivo antitumor effects of doxorubicin loaded with bacterial magnetosomes (DBMs) on H22 cells: the magnetic bio-nanoparticles as drug carriers. Cancer Lett. 2007;258(1):109–117. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

