TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection
- PMID: 30626688
- PMCID: PMC6401451
- DOI: 10.1128/JVI.01815-18
TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection
Abstract
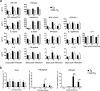
Transmembrane serine protease TMPRSS2 activates the spike protein of highly pathogenic human coronaviruses such as severe acute respiratory syndrome-related coronavirus (SARS-CoV) and Middle East respiratory syndrome-related coronavirus (MERS-CoV). In vitro, activation induces virus-cell membrane fusion at the cell surface. However, the roles of TMPRSS2 during coronavirus infection in vivo are unclear. Here, we used animal models of SARS-CoV and MERS-CoV infection to investigate the role of TMPRSS2. Th1-prone C57BL/6 mice and TMPRSS2-knockout (KO) mice were used for SARS-CoV infection, and transgenic mice expressing the human MERS-CoV receptor DPP4 (hDPP4-Tg mice) and TMPRSS2-KO hDPP4-Tg mice were used for MERS-CoV infection. After experimental infection, TMPRSS2-deficient mouse strains showed reduced body weight loss and viral kinetics in the lungs. Lack of TMPRSS2 affected the primary sites of infection and virus spread within the airway, accompanied by less severe immunopathology. However, TMPRSS2-KO mice showed weakened inflammatory chemokine and/or cytokine responses to intranasal stimulation with poly(I·C), a Toll-like receptor 3 agonist. In conclusion, TMPRSS2 plays a crucial role in viral spread within the airway of murine models infected by SARS-CoV and MERS-CoV and in the resulting immunopathology.IMPORTANCE Broad-spectrum antiviral drugs against highly pathogenic coronaviruses and other emerging viruses are desirable to enable a rapid response to pandemic threats. Transmembrane protease serine type 2 (TMPRSS2), a protease belonging to the type II transmembrane serine protease family, cleaves the coronavirus spike protein, making it a potential therapeutic target for coronavirus infections. Here, we examined the role of TMPRSS2 using animal models of SARS-CoV and MERS-CoV infection. The results suggest that lack of TMPRSS2 in the airways reduces the severity of lung pathology after infection by SARS-CoV and MERS-CoV. Taken together, the results will facilitate development of novel targets for coronavirus therapy.
Keywords: MERS-CoV; SARS-CoV; TMPRSS2; animal model; immunopathology.
Copyright © 2019 American Society for Microbiology.
Figures

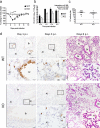

Similar articles
-
Acute Respiratory Infection in Human Dipeptidyl Peptidase 4-Transgenic Mice Infected with Middle East Respiratory Syndrome Coronavirus.J Virol. 2019 Mar 5;93(6):e01818-18. doi: 10.1128/JVI.01818-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626685 Free PMC article.
-
The TMPRSS2 Inhibitor Nafamostat Reduces SARS-CoV-2 Pulmonary Infection in Mouse Models of COVID-19.mBio. 2021 Aug 31;12(4):e0097021. doi: 10.1128/mBio.00970-21. Epub 2021 Aug 3. mBio. 2021. PMID: 34340553 Free PMC article.
-
Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2.J Virol. 2010 Dec;84(24):12658-64. doi: 10.1128/JVI.01542-10. Epub 2010 Oct 6. J Virol. 2010. PMID: 20926566 Free PMC article.
-
Molecular mechanisms for understanding the association between TMPRSS2 and beta coronaviruses SARS-CoV-2, SARS-CoV and MERS-CoV infection: scoping review.Arch Microbiol. 2021 Dec 25;204(1):77. doi: 10.1007/s00203-021-02727-3. Arch Microbiol. 2021. PMID: 34953136 Free PMC article. Review.
-
Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research.Antiviral Res. 2013 Dec;100(3):605-14. doi: 10.1016/j.antiviral.2013.09.028. Epub 2013 Oct 8. Antiviral Res. 2013. PMID: 24121034 Free PMC article. Review.
Cited by
-
Middle East Respiratory Syndrome Coronavirus Could be a Priority Pathogen to Cause Public Health Emergency: Noticeable Features and Counteractive Measures.Environ Health Insights. 2024 Aug 15;18:11786302241271545. doi: 10.1177/11786302241271545. eCollection 2024. Environ Health Insights. 2024. PMID: 39156879 Free PMC article.
-
Long COVID Definition, Symptoms, Risk Factors, Epidemiology and Autoimmunity: A Narrative Review.Am J Med Open. 2024 Feb 14;11:100068. doi: 10.1016/j.ajmo.2024.100068. eCollection 2024 Jun. Am J Med Open. 2024. PMID: 39034937 Free PMC article.
-
Computational screening combined with well-tempered metadynamics simulations identifies potential TMPRSS2 inhibitors.Sci Rep. 2024 Jul 13;14(1):16197. doi: 10.1038/s41598-024-65296-7. Sci Rep. 2024. PMID: 39003338 Free PMC article.
-
Novel proteolytic activation of Ebolavirus glycoprotein GP by TMPRSS2 and cathepsin L at an uncharted position can compensate for furin cleavage.Virus Res. 2024 Sep;347:199430. doi: 10.1016/j.virusres.2024.199430. Epub 2024 Jul 8. Virus Res. 2024. PMID: 38964470 Free PMC article.
-
Role of marine natural products in the development of antiviral agents against SARS-CoV-2: potential and prospects.Mar Life Sci Technol. 2024 Feb 21;6(2):280-297. doi: 10.1007/s42995-023-00215-9. eCollection 2024 May. Mar Life Sci Technol. 2024. PMID: 38827130 Review.
References
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ, SARS Working Group. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348:1953–1966. doi:10.1056/NEJMoa030781. - DOI - PubMed
-
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976. doi:10.1056/NEJMoa030747. - DOI - PubMed
-
- Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, Nicholls J, Yee WKS, Yan WW, Cheung MT, Cheng VCC, Chan KH, Tsang DNC, Yung RWH, Ng TK, Yuen KY. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325. doi:10.1016/S0140-6736(03)13077-2. - DOI - PMC - PubMed
-
- Zhong NS, Zheng BJ, Li YM, Poon LLM, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ, Xu J, Li DX, Yuen KY, Peiris JSM, Guan Y. 2003. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet 362:1353–1358. doi:10.1016/S0140-6736(03)14630-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

