Efficacy and safety of PAC-14028 cream - a novel, topical, nonsteroidal, selective TRPV1 antagonist in patients with mild-to-moderate atopic dermatitis: a phase IIb randomized trial
- PMID: 30623408
- PMCID: PMC6850419
- DOI: 10.1111/bjd.17455
Efficacy and safety of PAC-14028 cream - a novel, topical, nonsteroidal, selective TRPV1 antagonist in patients with mild-to-moderate atopic dermatitis: a phase IIb randomized trial
Abstract
Background: Transient receptor potential vanilloid subfamily, member 1 (TRPV1) may play an important role in pruritus and inflammation induction in atopic dermatitis (AD). The treatment effect of TRPV1 antagonist via topical application in patients with AD remains unknown.
Objectives: To assess the clinical efficacy and safety of PAC-14028, a TRPV1 antagonist, via topical application in patients with AD.
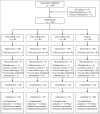
Methods: In this 8-week, phase IIb, randomized, double-blind, multicentre, vehicle-controlled study, patients with mild-to-moderate AD were randomized to receive PAC-14028 cream 0·1%, 0·3%, 1·0% or vehicle cream twice daily. The primary efficacy end point was the Investigator's Global Assessment (IGA) success rate defined as the percentage of patients with an IGA score of 0 or 1 at week 8. The secondary efficacy end points included the severity Scoring of Atopic Dermatitis (SCORAD) index and Eczema Area and Severity Index (EASI) 75/90.
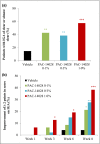
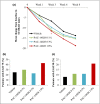
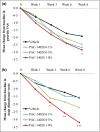
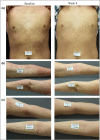
Results: A total of 194 patients were enrolled. IGA success rates at week 8 were 14·58% for vehicle cream, 42·55% for PAC-14028 cream 0·1% (P = 0·0025 vs. vehicle), 38·30% for PAC-14028 cream 0·3% (P = 0·0087 vs. vehicle) and 57·45% for PAC-14028 cream 1·0% (P < 0·001 vs. vehicle). In particular, statistically significant differences were found between the vehicle and treatment groups in the IGA success rates with two-grade improvement. The SCORAD index, EASI 75/90, sleep disturbance score and pruritus visual analogue scale showed a trend towards improvement. No significant safety issues were reported.
Conclusions: PAC-14028 cream may be an effective and safe treatment modality for the treatment of patients with mild-to-moderate AD.
© 2019 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures
Comment in
-
Novel therapeutic approach with PAC-14028 cream, a TRPV1 antagonist, for patients with mild-to-moderate atopic dermatitis.Br J Dermatol. 2019 May;180(5):971-972. doi: 10.1111/bjd.17777. Br J Dermatol. 2019. PMID: 31025744 No abstract available.
Similar articles
-
Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: Phase 3, randomized, vehicle-controlled study (CAPTAIN-AD).J Allergy Clin Immunol. 2022 Apr;149(4):1340-1347.e4. doi: 10.1016/j.jaci.2021.09.024. Epub 2021 Oct 2. J Allergy Clin Immunol. 2022. PMID: 34606832 Clinical Trial.
-
Efficacy, Safety, and Long-Term Disease Control of Ruxolitinib Cream Among Adolescents with Atopic Dermatitis: Pooled Results from Two Randomized Phase 3 Studies.Am J Clin Dermatol. 2024 Jul;25(4):669-683. doi: 10.1007/s40257-024-00855-2. Epub 2024 May 2. Am J Clin Dermatol. 2024. PMID: 38698175 Free PMC article. Clinical Trial.
-
Once-Daily Crisaborole Ointment, 2%, as a Long-Term Maintenance Treatment in Patients Aged ≥ 3 Months with Mild-to-Moderate Atopic Dermatitis: A 52-Week Clinical Study.Am J Clin Dermatol. 2023 Jul;24(4):623-635. doi: 10.1007/s40257-023-00780-w. Epub 2023 May 15. Am J Clin Dermatol. 2023. PMID: 37184828 Free PMC article. Clinical Trial.
-
Topical pimecrolimus: a review of its clinical potential in the management of atopic dermatitis.Drugs. 2002;62(5):817-40. doi: 10.2165/00003495-200262050-00007. Drugs. 2002. PMID: 11929333 Review.
-
Ruxolitinib Cream 1.5%: A Review in Mild to Moderate Atopic Dermatitis.Am J Clin Dermatol. 2023 Jan;24(1):143-151. doi: 10.1007/s40257-022-00748-2. Epub 2022 Dec 20. Am J Clin Dermatol. 2023. PMID: 36538235 Free PMC article. Review.
Cited by
-
A Comprehensive Review on the Regulatory Action of TRP Channels: A Potential Therapeutic Target for Nociceptive Pain.Neurosci Insights. 2023 Dec 24;18:26331055231220340. doi: 10.1177/26331055231220340. eCollection 2023. Neurosci Insights. 2023. PMID: 38146332 Free PMC article. Review.
-
Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation.Front Immunol. 2020 Oct 23;11:590261. doi: 10.3389/fimmu.2020.590261. eCollection 2020. Front Immunol. 2020. PMID: 33193423 Free PMC article. Review.
-
Molecular mechanisms of pruritus in prurigo nodularis.Front Immunol. 2023 Nov 23;14:1301817. doi: 10.3389/fimmu.2023.1301817. eCollection 2023. Front Immunol. 2023. PMID: 38077377 Free PMC article. Review.
-
Advances in TRP channel drug discovery: from target validation to clinical studies.Nat Rev Drug Discov. 2022 Jan;21(1):41-59. doi: 10.1038/s41573-021-00268-4. Epub 2021 Sep 15. Nat Rev Drug Discov. 2022. PMID: 34526696 Free PMC article. Review.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
References
-
- Nygaard U, Deleuran M, Vestergaard C. Emerging treatment options in atopic dermatitis: topical therapies. Dermatology 2017; 233:333–43. - PubMed
-
- Ring J. Atopic Dermatitis. Heidelberg: Springer International Publishing, 2016.
-
- Bieber T. Atopic dermatitis. N Engl J Med 2008; 358:1483–94. - PubMed
-
- Beattie PE, Lewis‐Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol 2006; 155:145–51. - PubMed
-
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol 2017; 140:633–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

