Co-Delivery of Timolol and Brimonidine with a Polymer Thin-Film Intraocular Device
- PMID: 30615539
- PMCID: PMC6450452
- DOI: 10.1089/jop.2018.0096
Co-Delivery of Timolol and Brimonidine with a Polymer Thin-Film Intraocular Device
Abstract
Purpose: We developed a polycaprolactone (PCL) co-delivery implant that achieves zero-order release of 2 ocular hypotensive agents, timolol maleate and brimonidine tartrate. We also demonstrate intraocular pressure (IOP)-lowering effects of the implant for 3 months in vivo.
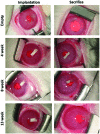
Methods: Two PCL thin-film compartments were attached to form a V-shaped co-delivery device using film thicknesses of ∼40 and 20 μm for timolol and brimonidine compartments, respectively. In vitro release kinetics were measured in pH- and temperature-controlled fluid chambers. Empty or drug-loaded devices were implanted intracamerally in normotensive rabbits for up to 13 weeks with weekly measurements of IOP. For ocular concentrations, rabbits were euthanized at 4, 8, or 13 weeks, aqueous fluid was collected, and ocular tissues were dissected. Drug concentrations were measured by liquid chromatography-tandem mass spectrometry.
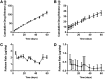
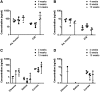
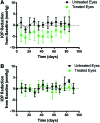
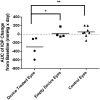
Results: In vitro studies show zero-order release kinetics for both timolol (1.75 μg/day) and brimonidine (0.48 μg/day) for up to 60 days. In rabbit eyes, the device achieved an average aqueous fluid concentration of 98.1 ± 68.3 ng/mL for timolol and 5.5 ± 3.6 ng/mL for brimonidine. Over 13 weeks, the drug-loaded co-delivery device resulted in a statistically significant cumulative reduction in IOP compared to untreated eyes (P < 0.05) and empty-device eyes (P < 0.05).
Conclusions: The co-delivery device demonstrated a zero-order release profile in vitro for 2 hypotensive agents over 60 days. In vivo, the device led to significant cumulative IOP reduction of 3.4 ± 1.6 mmHg over 13 weeks. Acceptable ocular tolerance was seen, and systemic drug levels were unmeasurable.
Keywords: brimonidine; co-delivery; controlled release; drug delivery systems; glaucoma; timolol.
Conflict of interest statement
T.A.D. is a co-founder of Zordera and is on the advisory board for Santen; R.B.B. receives a research grant from Genentech, receives consulting fees from Ribomic, Inc. and AntriaBio, is a co-founder of Zordera, is on the advisory board for Santen, and is on the advisory board for Sandoz, Biotime, and Quark. The remaining authors have no conflict of interests.
Figures


Similar articles
-
Multicenter, Randomized, Investigator-Masked Study Comparing Brimonidine Tartrate 0.1% and Timolol Maleate 0.5% as Adjunctive Therapies to Prostaglandin Analogues in Normal-Tension Glaucoma.Adv Ther. 2017 Jun;34(6):1438-1448. doi: 10.1007/s12325-017-0552-5. Epub 2017 May 15. Adv Ther. 2017. PMID: 28508306 Clinical Trial.
-
Comparison of the ocular hypotensive effect of brimonidine, dorzolamide, latanoprost, or artificial tears added to timolol in glaucomatous monkey eyes.J Glaucoma. 2000 Dec;9(6):458-62. doi: 10.1097/00061198-200012000-00007. J Glaucoma. 2000. PMID: 11131752
-
Effects of 0.2% brimonidine and 0.2% brimonidine-0.5% timolol on intraocular pressure and pupil size in normal equine eyes.Equine Vet J. 2017 Nov;49(6):810-814. doi: 10.1111/evj.12695. Epub 2017 Jun 5. Equine Vet J. 2017. PMID: 28470857 Free PMC article.
-
Topical brimonidine 0.2%/timolol 0.5% ophthalmic solution: in glaucoma and ocular hypertension.Drugs Aging. 2006;23(9):753-61. doi: 10.2165/00002512-200623090-00005. Drugs Aging. 2006. PMID: 17020399 Review.
-
Design and Development of Ophthalmic Liposomes from the QbD Perspective.Curr Pharm Des. 2024;30(30):2364-2377. doi: 10.2174/0113816128302570240627113909. Curr Pharm Des. 2024. PMID: 39021195 Review.
Cited by
-
Considerations for Polymers Used in Ocular Drug Delivery.Front Med (Lausanne). 2022 Jan 28;8:787644. doi: 10.3389/fmed.2021.787644. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35155469 Free PMC article. Review.
-
A long-acting formulation of rifabutin is effective for prevention and treatment of Mycobacterium tuberculosis.Nat Commun. 2022 Aug 8;13(1):4455. doi: 10.1038/s41467-022-32043-3. Nat Commun. 2022. PMID: 35941109 Free PMC article.
-
Nanostructure-Mediated Transport of Therapeutics through Epithelial Barriers.Int J Mol Sci. 2024 Jun 28;25(13):7098. doi: 10.3390/ijms25137098. Int J Mol Sci. 2024. PMID: 39000205 Free PMC article. Review.
-
Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma.Polymers (Basel). 2023 Mar 9;15(6):1373. doi: 10.3390/polym15061373. Polymers (Basel). 2023. PMID: 36987154 Free PMC article. Review.
-
Biodegradable Polymer-Based Drug-Delivery Systems for Ocular Diseases.Int J Mol Sci. 2023 Aug 19;24(16):12976. doi: 10.3390/ijms241612976. Int J Mol Sci. 2023. PMID: 37629157 Free PMC article. Review.
References
-
- Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., and Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 121:2081–2090, 2014 - PubMed
-
- Olthoff C.M.G., Schouten J.S.A.G., Van De Borne B.W., and Webers C.A.B. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology. 112:953–961, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

