Hybrid Inhibitors of Malarial Dihydrofolate Reductase with Dual Binding Modes That Can Forestall Resistance
- PMID: 30613332
- PMCID: PMC6295868
- DOI: 10.1021/acsmedchemlett.8b00389
Hybrid Inhibitors of Malarial Dihydrofolate Reductase with Dual Binding Modes That Can Forestall Resistance
Abstract
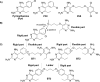
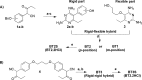
The S108N mutation of dihydrofolate reductase (DHFR) renders Plasmodium falciparum malaria parasites resistant to pyrimethamine through steric clash with the rigid side chain of the inhibitor. Inhibitors with flexible side chains can avoid this clash and retain effectiveness against the mutant. However, other mutations such as N108S reversion confer resistance to flexible inhibitors. We designed and synthesized hybrid inhibitors with two structural types in a single molecule, which are effective against both wild-type and multiple mutants of P. falciparum through their selective target binding, as demonstrated by X-ray crystallography. Furthermore, the hybrid inhibitors can forestall the emergence of new resistant mutants, as shown by selection of mutants resistant to hybrid compound BT1 from a diverse PfDHFR random mutant library expressed in a surrogate bacterial system. These results show that it is possible to develop effective antifolate antimalarials to which the range of parasite resistance mutations is greatly reduced.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
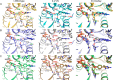
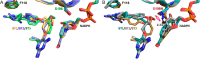
Similar articles
-
Pyrimethamine analogs as strong inhibitors of double and quadruple mutants of dihydrofolate reductase in human malaria parasites.Org Biomol Chem. 2003 Mar 21;1(6):960-4. doi: 10.1039/b211636g. Org Biomol Chem. 2003. PMID: 12929634
-
Malarial (Plasmodium falciparum) dihydrofolate reductase-thymidylate synthase: structural basis for antifolate resistance and development of effective inhibitors.Parasitology. 2005 Mar;130(Pt 3):249-59. doi: 10.1017/s003118200400664x. Parasitology. 2005. PMID: 15796007 Review.
-
Conflicting requirements of Plasmodium falciparum dihydrofolate reductase mutations conferring resistance to pyrimethamine-WR99210 combination.Antimicrob Agents Chemother. 2007 Dec;51(12):4356-60. doi: 10.1128/AAC.00577-07. Epub 2007 Sep 17. Antimicrob Agents Chemother. 2007. PMID: 17875995 Free PMC article.
-
Molecular dynamics of interactions between rigid and flexible antifolates and dihydrofolate reductase from pyrimethamine-sensitive and pyrimethamine-resistant Plasmodium falciparum.Chem Biol Drug Des. 2014 Oct;84(4):450-61. doi: 10.1111/cbdd.12334. Epub 2014 Jun 4. Chem Biol Drug Des. 2014. PMID: 24716467
-
Development of 2,4-diaminopyrimidines as antimalarials based on inhibition of the S108N and C59R+S108N mutants of dihydrofolate reductase from pyrimethamine-resistant Plasmodium falciparum.J Med Chem. 2002 Mar 14;45(6):1244-52. doi: 10.1021/jm010131q. J Med Chem. 2002. PMID: 11881993
Cited by
-
Deep mutational scanning of Pneumocystis jirovecii dihydrofolate reductase reveals allosteric mechanism of resistance to an antifolate.PLoS Genet. 2024 Apr 29;20(4):e1011252. doi: 10.1371/journal.pgen.1011252. eCollection 2024 Apr. PLoS Genet. 2024. PMID: 38683847 Free PMC article.
-
Discovery of rigid biphenyl Plasmodium falciparum DHFR inhibitors using a fragment linking strategy.RSC Med Chem. 2023 Jul 24;14(9):1755-1766. doi: 10.1039/d3md00242j. eCollection 2023 Sep 19. RSC Med Chem. 2023. PMID: 37731689 Free PMC article.
-
Roles of Virtual Screening and Molecular Dynamics Simulations in Discovering and Understanding Antimalarial Drugs.Int J Mol Sci. 2023 May 26;24(11):9289. doi: 10.3390/ijms24119289. Int J Mol Sci. 2023. PMID: 37298256 Free PMC article. Review.
-
Assay Development and Identification of the First Plasmodium falciparum 7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase Inhibitors.Molecules. 2022 May 30;27(11):3515. doi: 10.3390/molecules27113515. Molecules. 2022. PMID: 35684452 Free PMC article.
-
A Dual, Systematic Approach to Malaria Diagnostic Biomarker Discovery.Clin Infect Dis. 2022 Jan 7;74(1):40-51. doi: 10.1093/cid/ciab251. Clin Infect Dis. 2022. PMID: 34718455 Free PMC article.
References
-
- Rabinovich R. N.; Drakeley C.; Djimde A. A.; Hall B. F.; Hay S. I.; Hemingway J.; Kaslow D. C.; Noor A.; Okumu F.; Steketee R.; Tanner M.; Wells T. N. C.; Whittaker M. A.; Winzeler E. A.; Wirth D. F.; Whitfield K.; Alonso P. L. malERA: An updated research agenda for malaria elimination and eradication. PLoS Med. 2017, 14, e1002456.10.1371/journal.pmed.1002456. - DOI - PMC - PubMed
-
- Imwong M.; Suwannasin K.; Kunasol C.; Sutawong K.; Mayxay M.; Rekol H.; Smithuis F. M.; Hlaing T. M.; Tun K. M.; van der Pluijm R. W.; Tripura R.; Miotto O.; Menard D.; Dhorda M.; Day N. P. J.; White N. J.; Dondorp A. M. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect. Dis. 2017, 17, 491–497. 10.1016/S1473-3099(17)30048-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information

