Clinical significance and biological role of cancer-derived Type I collagen in lung and esophageal cancers
- PMID: 30604926
- PMCID: PMC6360244
- DOI: 10.1111/1759-7714.12947
Clinical significance and biological role of cancer-derived Type I collagen in lung and esophageal cancers
Abstract
Background: Extracellular matrix (ECM) is remodeled during carcinogenesis. An abundant constituent of ECM is collagen. Type I collagen is secreted by fibroblasts, is important for tumor growth and epithelial-mesenchymal transition, and may also be secreted by cancer cells. However, the role and function of cancer-derived Type I collagen in the tumor microenvironment remains unclear.
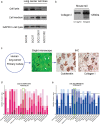
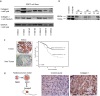
Methods: We used immunohistochemistry and Western blot to detect Type I collagen expression in non-small cell lung cancer (NSCLC) and esophageal squamous cell carcinoma (ESCC) cell lines, respectively. We assessed the migration and adhesion capability of these cells in vivo by inhibiting Type I collagen in tumors. Relevant data were extracted from a large cohort study of The Cancer Genome Atlas to analyze messenger RNA levels. Protein expression of Type I collagen was further determined in tumor tissues of patients using tissue microarray.
Results: Cancer cell lines secreted Type I collagen. The molecular weight of cancer-derived Type I collagen was different from that secreted by cancer-associated fibroblasts and normal fibroblasts. Expression levels of COL1A1 and COL1A2 (subtypes of Type I collagen) messenger RNA in NSCLC and ESCC tumors were higher than in normal tissues, but were not associated with tumor node metastasis stages. Low expression of Type I collagen was significantly associated with poor overall survival and cancer cell differentiation.
Conclusion: NSCLC and ESCC cells could produce Type I collagen endogenously, revealing the potential functions of Type I collagen in cancer development. Cancer-derived Type I collagen was associated with overall survival and cancer cell differentiation.
Keywords: Type I collagen; esophagus cancer; extracellular matrix; non-small cell lung cancer; tumor microenvironment.
© 2019 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures
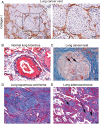

 H,
H,  M,
M,  L,
L,  Not,
Not,  H,
H,  M,
M,  L,
L,  Not.
Not.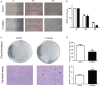
 Control,
Control,  C inhibitor.
C inhibitor.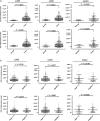
Similar articles
-
Downregulation of the TGFβ Pseudoreceptor BAMBI in Non-Small Cell Lung Cancer Enhances TGFβ Signaling and Invasion.Cancer Res. 2016 Jul 1;76(13):3785-801. doi: 10.1158/0008-5472.CAN-15-1326. Epub 2016 May 17. Cancer Res. 2016. PMID: 27197161
-
Comparison of cofilin‑1 and Twist‑1 protein expression in human non‑small cell lung cancer tissues.Oncol Rep. 2019 Aug;42(2):805-816. doi: 10.3892/or.2019.7193. Epub 2019 Jun 10. Oncol Rep. 2019. PMID: 31233187
-
TRIM47 overexpression is a poor prognostic factor and contributes to carcinogenesis in non-small cell lung carcinoma.Oncotarget. 2017 Apr 4;8(14):22730-22740. doi: 10.18632/oncotarget.15188. Oncotarget. 2017. PMID: 28186994 Free PMC article.
-
Roles of Lipid Profiles in Human Non-Small Cell Lung Cancer.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211041472. doi: 10.1177/15330338211041472. Technol Cancer Res Treat. 2021. PMID: 34569862 Free PMC article. Review.
-
Targeting type I collagen for cancer treatment.Int J Cancer. 2022 Sep 1;151(5):665-683. doi: 10.1002/ijc.33985. Epub 2022 Mar 8. Int J Cancer. 2022. PMID: 35225360 Review.
Cited by
-
RhoBTB3 Regulates Proliferation and Invasion of Breast Cancer Cells via Col1a1.Mol Cells. 2022 Sep 30;45(9):631-639. doi: 10.14348/molcells.2022.2037. Epub 2022 Jun 14. Mol Cells. 2022. PMID: 35698915 Free PMC article.
-
The Functional Role of Extracellular Matrix Proteins in Cancer.Cancers (Basel). 2022 Jan 4;14(1):238. doi: 10.3390/cancers14010238. Cancers (Basel). 2022. PMID: 35008401 Free PMC article. Review.
-
Rigid Tissue Increases Cytoplasmic pYAP Expression in Pre-Malignant Stage of Lung Squamous Cell Carcinoma (SCC) In Vivo.Curr Issues Mol Biol. 2022 Sep 29;44(10):4528-4539. doi: 10.3390/cimb44100310. Curr Issues Mol Biol. 2022. PMID: 36286025 Free PMC article.
-
The clinical significance of collagen family gene expression in esophageal squamous cell carcinoma.PeerJ. 2019 Oct 4;7:e7705. doi: 10.7717/peerj.7705. eCollection 2019. PeerJ. 2019. PMID: 31598423 Free PMC article.
-
Mass Spectrometry Imaging for Reliable and Fast Classification of Non-Small Cell Lung Cancer Subtypes.Cancers (Basel). 2020 Sep 21;12(9):2704. doi: 10.3390/cancers12092704. Cancers (Basel). 2020. PMID: 32967325 Free PMC article.
References
-
- Palmieri D, Astigiano S, Barbieri O et al Procollagen I COOH‐terminal fragment induces VEGF‐A and CXCR4 expression in breast carcinoma cells. Exp Cell Res 2008; 314: 2289–98. - PubMed
-
- Cretu A, Brooks PC. Impact of the non‐cellular tumor microenvironment on metastasis: Potential therapeutic and imaging opportunities. J Cell Physiol 2007; 213: 391–402. - PubMed
-
- Duesberg P, Mandrioli D, McCormack A, Nicholson JM. Is carcinogenesis a form of speciation? Cell Cycle 2011; 10: 2100–14. - PubMed
-
- Friedl P, Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011; 147: 992–1009. - PubMed
-
- Kokenyesi R, Murray KP, Benshushan A, Huntley ED, Kao MS. Invasion of interstitial matrix by a novel cell line from primary peritoneal carcinosarcoma, and by established ovarian carcinoma cell lines: Role of cell‐matrix adhesion molecules, proteinases, and E‐cadherin expression. Gynecol Oncol 2003; 89: 60–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

