A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis
- PMID: 30582442
- PMCID: PMC6325767
- DOI: 10.1161/CIRCRESAHA.118.313234
A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis
Abstract
Rationale: Several studies have suggested a role for the gut microbiota in inflammation and atherogenesis. A causal relation relationship between gut microbiota, inflammation, and atherosclerosis has not been explored previously.
Objective: Here, we investigated whether a proinflammatory microbiota from Caspase1-/- ( Casp1-/-) mice accelerates atherogenesis in Ldlr-/- mice.
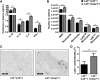
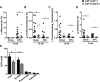
Method and results: We treated female Ldlr-/- mice with antibiotics and subsequently transplanted them with fecal microbiota from Casp1-/- mice based on a cohousing approach. Autologous transplantation of fecal microbiota of Ldlr-/- mice served as control. Mice were cohoused for 8 or 13 weeks and fed chow or high-fat cholesterol-rich diet. Fecal samples were collected, and factors related to inflammation, metabolism, intestinal health, and atherosclerotic phenotypes were measured. Unweighted Unifrac distances of 16S rDNA (ribosomal DNA) sequences confirmed the introduction of the Casp1-/- and Ldlr-/- microbiota into Ldlr-/- mice (referred to as Ldlr-/-( Casp1-/-) or Ldlr-/-( Ldlr-/-) mice). Analysis of atherosclerotic lesion size in the aortic root demonstrated a significant 29% increase in plaque size in 13-week high-fat cholesterol-fed Ldlr-/-( Casp1-/-) mice compared with Ldlr-/-( Ldlr-/-) mice. We found increased numbers of circulating monocytes and neutrophils and elevated proinflammatory cytokine levels in plasma in high-fat cholesterol-fed Ldlr-/-( Casp1-/-) compared with Ldlr-/-( Ldlr-/-) mice. Neutrophil accumulation in the aortic root of Ldlr-/-( Casp1-/-) mice was enhanced compared with Ldlr-/-( Ldlr-/-) mice. 16S-rDNA-encoding sequence analysis in feces identified a significant reduction in the short-chain fatty acid-producing taxonomies Akkermansia, Christensenellaceae, Clostridium, and Odoribacter in Ldlr-/-( Casp1-/-) mice. Consistent with these findings, cumulative concentrations of the anti-inflammatory short-chain fatty acids propionate, acetate and butyrate in the cecum were significantly reduced in 13-week high-fat cholesterol-fed Ldlr-/-( Casp1-/-) compared with Ldlr-/-( Ldlr-/-) mice.
Conclusions: Introduction of the proinflammatory Casp1-/- microbiota into Ldlr-/- mice enhances systemic inflammation and accelerates atherogenesis.
Keywords: atherosclerosis; cholesterol; diet; fatty acids, volatile; feces; inflammation.
Figures


Comment in
-
Knights in Shining Armor.Circ Res. 2019 Jan 4;124(1):12-14. doi: 10.1161/CIRCRESAHA.118.314246. Circ Res. 2019. PMID: 30605411 No abstract available.
Similar articles
-
Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe-/- mice.Atherosclerosis. 2018 Jan;268:117-126. doi: 10.1016/j.atherosclerosis.2017.11.023. Epub 2017 Nov 24. Atherosclerosis. 2018. PMID: 29202334
-
Lack of myeloid Fatp1 increases atherosclerotic lesion size in Ldlr-/- mice.Atherosclerosis. 2017 Nov;266:182-189. doi: 10.1016/j.atherosclerosis.2017.10.009. Epub 2017 Oct 7. Atherosclerosis. 2017. PMID: 29035781 Free PMC article.
-
Nuclear factor E2-related factor 2 deficiency impairs atherosclerotic lesion development but promotes features of plaque instability in hypercholesterolaemic mice.Cardiovasc Res. 2019 Jan 1;115(1):243-254. doi: 10.1093/cvr/cvy143. Cardiovasc Res. 2019. PMID: 29917052
-
The gut microbiome and cardiovascular disease: current knowledge and clinical potential.Am J Physiol Heart Circ Physiol. 2019 Nov 1;317(5):H923-H938. doi: 10.1152/ajpheart.00376.2019. Epub 2019 Aug 30. Am J Physiol Heart Circ Physiol. 2019. PMID: 31469291 Review.
-
Mutual Interplay of Host Immune System and Gut Microbiota in the Immunopathology of Atherosclerosis.Int J Mol Sci. 2020 Nov 19;21(22):8729. doi: 10.3390/ijms21228729. Int J Mol Sci. 2020. PMID: 33227973 Free PMC article. Review.
Cited by
-
Recovering intestinal redox homeostasis to resolve systemic inflammation for preventing remote myocardial injury by oral fullerenes.Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2311673120. doi: 10.1073/pnas.2311673120. Epub 2023 Dec 18. Proc Natl Acad Sci U S A. 2023. PMID: 38109541 Free PMC article.
-
Mechanisms, therapeutic implications, and methodological challenges of gut microbiota and cardiovascular diseases: a position paper by the ESC Working Group on Coronary Pathophysiology and Microcirculation.Cardiovasc Res. 2022 Dec 29;118(16):3171-3182. doi: 10.1093/cvr/cvac057. Cardiovasc Res. 2022. PMID: 35420126 Free PMC article. Review.
-
The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation.Front Cell Infect Microbiol. 2022 Jun 20;12:903570. doi: 10.3389/fcimb.2022.903570. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35795187 Free PMC article. Review.
-
Mechanisms shared between cancer, heart failure, and targeted anti-cancer therapies.Cardiovasc Res. 2023 Feb 3;118(18):3451-3466. doi: 10.1093/cvr/cvac132. Cardiovasc Res. 2023. PMID: 36004495 Free PMC article. Review.
-
Multi-Pharmacology of Berberine in Atherosclerosis and Metabolic Diseases: Potential Contribution of Gut Microbiota.Front Pharmacol. 2021 Jul 9;12:709629. doi: 10.3389/fphar.2021.709629. eCollection 2021. Front Pharmacol. 2021. PMID: 34305616 Free PMC article. Review.
References
-
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. - PubMed
-
- Ridker PM, Everett BM, Thuren T, et al. CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. - PubMed
-
- Schirmer M, Smeekens SP, Vlamakis H, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1897. doi: 10.1016/j.cell.2016.11.046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

