Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome
- PMID: 30555165
- PMCID: PMC6365492
- DOI: 10.1038/s41375-018-0312-9
Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome
Abstract
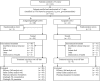
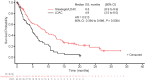
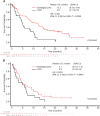
Glasdegib is a Hedgehog pathway inhibitor. This phase II, randomized, open-label, multicenter study (ClinicalTrials.gov, NCT01546038) evaluated the efficacy of glasdegib plus low-dose cytarabine (LDAC) in patients with acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome unsuitable for intensive chemotherapy. Glasdegib 100 mg (oral, QD) was administered continuously in 28-day cycles; LDAC 20 mg (subcutaneous, BID) was administered for 10 per 28 days. Patients (stratified by cytogenetic risk) were randomized (2:1) to receive glasdegib/LDAC or LDAC. The primary endpoint was overall survival. Eighty-eight and 44 patients were randomized to glasdegib/LDAC and LDAC, respectively. Median (80% confidence interval [CI]) overall survival was 8.8 (6.9-9.9) months with glasdegib/LDAC and 4.9 (3.5-6.0) months with LDAC (hazard ratio, 0.51; 80% CI, 0.39-0.67, P = 0.0004). Fifteen (17.0%) and 1 (2.3%) patients in the glasdegib/LDAC and LDAC arms, respectively, achieved complete remission (P < 0.05). Nonhematologic grade 3/4 all-causality adverse events included pneumonia (16.7%) and fatigue (14.3%) with glasdegib/LDAC and pneumonia (14.6%) with LDAC. Clinical efficacy was evident across patients with diverse mutational profiles. Glasdegib plus LDAC has a favorable benefit-risk profile and may be a promising option for AML patients unsuitable for intensive chemotherapy.
Conflict of interest statement
JEC received research funding and consulting honoraria from Pfizer, Novartis, Astellas, Daiichi, and Celgene. FHH received honoraria from Pfizer. WF participated in advisory boards for Amgen, Pfizer, Novartis, Jazz, and ARIAD/Incyte; has patents and royalties from Amgen; and received support for meeting attendance from Amgen, Gilead, GSO, Teva, and Jazz and research funding from Amgen and Pfizer. BDS served on an advisory board and was a consultant for Celgene, Jazz Pharmaceuticals, Novartis, and Pfizer. TR received research funding from Pfizer. PM served on an advisory board for Celgene, Jazz, Janssen, and Novartis and has received research funding from Pfizer and Celgene. DAP served on an advisory board for Agios, Celgene, Curis, Takeda, Servier, Jazz, and Gilead and has received research funding from Pfizer and Agios. AH, PD, and OO were investigators for this Pfizer-funded study and received study drugs provided by Pfizer. MH received research funding and honoraria from Pfizer. WWM, MNS, ADL, MZ, AO, and GC are employees of and own stock in Pfizer Inc.
Figures
Similar articles
-
Clinical benefit of glasdegib plus low-dose cytarabine in patients with de novo and secondary acute myeloid leukemia: long-term analysis of a phase II randomized trial.Ann Hematol. 2021 May;100(5):1181-1194. doi: 10.1007/s00277-021-04465-4. Epub 2021 Mar 19. Ann Hematol. 2021. PMID: 33740113 Free PMC article. Clinical Trial.
-
An evaluation of overall survival in patients with newly diagnosed acute myeloid leukemia and the relationship with glasdegib treatment and exposure.Cancer Chemother Pharmacol. 2020 Oct;86(4):451-459. doi: 10.1007/s00280-020-04132-x. Epub 2020 Sep 3. Cancer Chemother Pharmacol. 2020. PMID: 32885274 Free PMC article. Clinical Trial.
-
Survival outcomes and clinical benefit in patients with acute myeloid leukemia treated with glasdegib and low-dose cytarabine according to response to therapy.J Hematol Oncol. 2020 Jul 14;13(1):92. doi: 10.1186/s13045-020-00929-8. J Hematol Oncol. 2020. PMID: 32664995 Free PMC article.
-
Selection and management of older patients with acute myeloid leukemia treated with glasdegib plus low-dose cytarabine: expert panel review.Leuk Lymphoma. 2020 Dec;61(14):3287-3305. doi: 10.1080/10428194.2020.1817445. Epub 2020 Sep 24. Leuk Lymphoma. 2020. PMID: 32967493 Review.
-
Glasdegib: First Global Approval.Drugs. 2019 Feb;79(2):207-213. doi: 10.1007/s40265-018-1047-7. Drugs. 2019. PMID: 30666593 Review.
Cited by
-
The Role of Metabolism in the Development of Personalized Therapies in Acute Myeloid Leukemia.Front Oncol. 2021 May 19;11:665291. doi: 10.3389/fonc.2021.665291. eCollection 2021. Front Oncol. 2021. PMID: 34094959 Free PMC article. Review.
-
Targeting hedgehog-driven mechanisms of drug-resistant cancers.Front Mol Biosci. 2023 Oct 23;10:1286090. doi: 10.3389/fmolb.2023.1286090. eCollection 2023. Front Mol Biosci. 2023. PMID: 37954979 Free PMC article. Review.
-
Survival of patients with newly diagnosed high-grade myeloid neoplasms who do not meet standard trial eligibility.Haematologica. 2021 Aug 1;106(8):2114-2120. doi: 10.3324/haematol.2020.254938. Haematologica. 2021. PMID: 32646891 Free PMC article.
-
A Focus on Intermediate-Risk Acute Myeloid Leukemia: Sub-Classification Updates and Therapeutic Challenges.Cancers (Basel). 2022 Aug 28;14(17):4166. doi: 10.3390/cancers14174166. Cancers (Basel). 2022. PMID: 36077703 Free PMC article. Review.
-
Small molecule inhibitors targeting the cancers.MedComm (2020). 2022 Oct 13;3(4):e181. doi: 10.1002/mco2.181. eCollection 2022 Dec. MedComm (2020). 2022. PMID: 36254250 Free PMC article. Review.
References
-
- Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–24. doi: 10.1002/cncr.22496. - DOI - PubMed
-
- Burnett AK, Russell NH, Culligan D, Cavanagh J, Kell J, Wheatley K, et al. The addition of the farnesyl transferase inhibitor, tipifarnib, to low dose cytarabine does not improve outcome for older patients with AML. Br J Haematol. 2012;158:519–22. doi: 10.1111/j.1365-2141.2012.09165.x. - DOI - PubMed
-
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–7. doi: 10.1200/JCO.2011.38.9429. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

