Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission
- PMID: 30531964
- PMCID: PMC6286342
- DOI: 10.1038/s41467-018-07543-w
Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission
Abstract
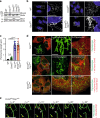
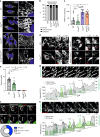
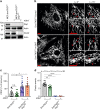
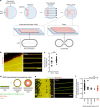
Dynamin-related protein 1 (Drp1) is essential for mitochondrial and peroxisomal fission. Recent studies propose that Drp1 does not sever but rather constricts mitochondrial membranes allowing dynamin 2 (Dnm2) to execute final scission. Here, we report that unlike Drp1, Dnm2 is dispensable for peroxisomal and mitochondrial fission, as these events occurred in Dnm2 knockout cells. Fission events were also observed in mouse embryonic fibroblasts lacking Dnm1, 2 and 3. Using reconstitution experiments on preformed membrane tubes, we show that Drp1 alone both constricts and severs membrane tubes. Scission required the membrane binding, self-assembling and GTPase activities of Drp1 and occurred on tubes up to 250 nm in radius. In contrast, Dnm2 exhibited severely restricted fission capacity with occasional severing of tubes below 50 nm in radius. We conclude that Drp1 has both membrane constricting and severing abilities and is the dominant dynamin performing mitochondrial and peroxisomal fission.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission.J Biol Chem. 2013 Sep 20;288(38):27584-27593. doi: 10.1074/jbc.M113.479873. Epub 2013 Aug 6. J Biol Chem. 2013. PMID: 23921378 Free PMC article.
-
Imaging Dynamin-Related Protein 1 (Drp1)-Mediated Mitochondrial Fission in Living Cells.Methods Mol Biol. 2020;2159:205-217. doi: 10.1007/978-1-0716-0676-6_16. Methods Mol Biol. 2020. PMID: 32529374
-
Quantifying Drp1-Mediated Mitochondrial Fission by Immunostaining in Fixed Cells.Methods Mol Biol. 2020;2159:197-204. doi: 10.1007/978-1-0716-0676-6_15. Methods Mol Biol. 2020. PMID: 32529373 Free PMC article.
-
Molecular Basis of Mitochondrial and Peroxisomal Division Machineries.Int J Mol Sci. 2020 Jul 30;21(15):5452. doi: 10.3390/ijms21155452. Int J Mol Sci. 2020. PMID: 32751702 Free PMC article. Review.
-
Dynamin assembly strategies and adaptor proteins in mitochondrial fission.Curr Biol. 2013 Oct 7;23(19):R891-9. doi: 10.1016/j.cub.2013.08.040. Curr Biol. 2013. PMID: 24112988 Free PMC article. Review.
Cited by
-
Mitochondrial function in development and disease.Dis Model Mech. 2021 Jun 1;14(6):dmm048912. doi: 10.1242/dmm.048912. Epub 2021 Jun 11. Dis Model Mech. 2021. PMID: 34114603 Free PMC article. Review.
-
Mitochondrial dynamics during spermatogenesis.J Cell Sci. 2020 Jul 16;133(14):jcs235937. doi: 10.1242/jcs.235937. J Cell Sci. 2020. PMID: 32675215 Free PMC article. Review.
-
Mitochondria-hubs for regulating cellular biochemistry: emerging concepts and networks.Open Biol. 2019 Aug 30;9(8):190126. doi: 10.1098/rsob.190126. Epub 2019 Aug 7. Open Biol. 2019. PMID: 31387448 Free PMC article.
-
Distinct Contributions of the Peroxisome-Mitochondria Fission Machinery During Sexual Development of the Fungus Podospora anserina.Front Microbiol. 2020 Apr 15;11:640. doi: 10.3389/fmicb.2020.00640. eCollection 2020. Front Microbiol. 2020. PMID: 32351478 Free PMC article.
-
Mitochondrial dysfunction is a key link involved in the pathogenesis of sick sinus syndrome: a review.Front Cardiovasc Med. 2024 Oct 29;11:1488207. doi: 10.3389/fcvm.2024.1488207. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39534498 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

