The MEK Inhibitor Trametinib Ameliorates Kidney Fibrosis by Suppressing ERK1/2 and mTORC1 Signaling
- PMID: 30530834
- PMCID: PMC6317609
- DOI: 10.1681/ASN.2018020209
The MEK Inhibitor Trametinib Ameliorates Kidney Fibrosis by Suppressing ERK1/2 and mTORC1 Signaling
Abstract
Background: During kidney fibrosis, a hallmark and promoter of CKD (regardless of the underlying renal disorder leading to CKD), the extracellular-regulated kinase 1/2 (ERK1/2) pathway, is activated and has been implicated in the detrimental differentiation and expansion of kidney fibroblasts. An ERK1/2 pathway inhibitor, trametinib, is currently used in the treatment of melanoma, but its efficacy in the setting of CKD and renal fibrosis has not been explored.
Methods: We investigated whether trametinib has antifibrotic effects in two mouse models of renal fibrosis-mice subjected to unilateral ureteral obstruction (UUO) or fed an adenine-rich diet-as well as in cultured primary human fibroblasts. We also used immunoblot analysis, immunohistochemical staining, and other tools to study underlying molecular mechanisms for antifibrotic effects.
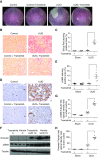
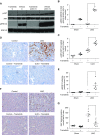
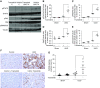
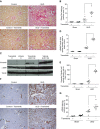
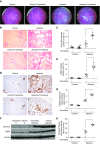
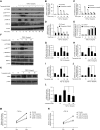
Results: Trametinib significantly attenuated collagen deposition and myofibroblast differentiation and expansion in UUO and adenine-fed mice. We also discovered that in injured kidneys, inhibition of the ERK1/2 pathway by trametinib ameliorated mammalian target of rapamycin complex 1 (mTORC1) activation, another key profibrotic signaling pathway. Trametinib also inhibited the ERK1/2 pathway in cultured primary human renal fibroblasts stimulated by application of TGF-β1, the major profibrotic cytokine, thereby suppressing downstream mTORC1 pathway activation. Additionally, trametinib reduced the expression of myofibroblast marker α-smooth muscle actin and the proliferation of renal fibroblasts, corroborating our in vivo data. Crucially, trametinib also significantly ameliorated renal fibrosis progression when administered to animals subsequent to myofibroblast activation.
Conclusions: Further study of trametinib as a potential candidate for the treatment of chronic renal fibrotic diseases of diverse etiologies is warranted.
Keywords: ERK1/2; Trametinib; UUO; chronic kidney disease; mTORC1; renal fibrosis.
Copyright © 2019 by the American Society of Nephrology.
Figures

Similar articles
-
Inhibition of the ERK1/2-mTORC1 axis ameliorates proteinuria and the fibrogenic action of transforming growth factor-β in Adriamycin-induced glomerulosclerosis.Kidney Int. 2019 Oct;96(4):927-941. doi: 10.1016/j.kint.2019.05.006. Epub 2019 May 22. Kidney Int. 2019. PMID: 31377057
-
Inhibition of spleen tyrosine kinase (syk) suppresses renal fibrosis through anti-inflammatory effects and down regulation of the MAPK-p38 pathway.Int J Biochem Cell Biol. 2016 May;74:135-44. doi: 10.1016/j.biocel.2016.03.001. Epub 2016 Mar 3. Int J Biochem Cell Biol. 2016. PMID: 26948651
-
Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species.Cell Signal. 2013 Nov;25(11):2198-209. doi: 10.1016/j.cellsig.2013.07.007. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872073
-
Trametinib: a novel signal transduction inhibitor for the treatment of metastatic cutaneous melanoma.Am J Health Syst Pharm. 2015 Jan 15;72(2):101-10. doi: 10.2146/ajhp140045. Am J Health Syst Pharm. 2015. PMID: 25550132 Review.
-
Therapeutic targets for treating fibrotic kidney diseases.Transl Res. 2015 Apr;165(4):512-30. doi: 10.1016/j.trsl.2014.07.010. Epub 2014 Aug 13. Transl Res. 2015. PMID: 25176603 Free PMC article. Review.
Cited by
-
Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway.Theranostics. 2020 Jul 25;10(20):9425-9442. doi: 10.7150/thno.43315. eCollection 2020. Theranostics. 2020. PMID: 32802201 Free PMC article.
-
Histone demethylase JMJD3 protects against renal fibrosis by suppressing TGFβ and Notch signaling and preserving PTEN expression.Theranostics. 2021 Jan 1;11(6):2706-2721. doi: 10.7150/thno.48679. eCollection 2021. Theranostics. 2021. PMID: 33456568 Free PMC article.
-
Rictor/mTORC2 signalling contributes to renal vascular endothelial-to-mesenchymal transition and renal allograft interstitial fibrosis by regulating BNIP3-mediated mitophagy.Clin Transl Med. 2024 May;14(5):e1686. doi: 10.1002/ctm2.1686. Clin Transl Med. 2024. PMID: 38769658 Free PMC article.
-
Basement membrane proteins in extracellular matrix characterize NF1 neurofibroma development and response to MEK inhibitor.J Clin Invest. 2023 Jun 15;133(12):e168227. doi: 10.1172/JCI168227. J Clin Invest. 2023. PMID: 37140985 Free PMC article.
-
Efficacy of Trametinib in Alleviating Cisplatin-Induced Acute Kidney Injury: Inhibition of Inflammation, Oxidative Stress, and Tubular Cell Death in a Mouse Model.Molecules. 2024 Jun 17;29(12):2881. doi: 10.3390/molecules29122881. Molecules. 2024. PMID: 38930946 Free PMC article.
References
-
- Leaf IA, Duffield JS: What can target kidney fibrosis? Nephrol Dial Transplant 32[Suppl 1]: i89–i97, 2017 - PubMed
-
- Nicholson ML, McCulloch TA, Harper SJ, Wheatley TJ, Edwards CM, Feehally J, et al. .: Early measurement of interstitial fibrosis predicts long-term renal function and graft survival in renal transplantation. Br J Surg 83: 1082–1085, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

