Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system
- PMID: 30524891
- PMCID: PMC6279341
- DOI: 10.1080/2162402X.2018.1500108
Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system
Abstract
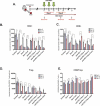
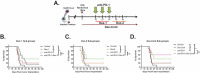
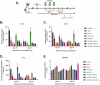
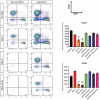
Immune checkpoint blockade targeting programmed cell death protein 1 (PD-1) is emerging as an important treatment strategy in a growing list of cancers, yet its clinical benefits are limited to a subset of patients. Further investigation of tumor-intrinsic predictors of response and how extrinsic factors, such as iatrogenic immunosuppression caused by conventional therapies, impact the efficacy of anti-PD-1 therapy are paramount. Given the widespread use of corticosteroids in cancer management and their immunosuppressive nature, this study sought to determine how corticosteroids influence anti-PD-1 responses and whether their effects were dependent on tumor location within the periphery versus central nervous system (CNS), which may have a more limiting immune environment. In well-established anti-PD-1-responsive murine tumor models, corticosteroid therapy resulted in systemic immune effects, including severe and persistent reductions in peripheral CD4+ and CD8 + T cells. Corticosteroid treatment was found to diminish the efficacy of anti-PD-1 therapy in mice bearing peripheral tumors with responses correlating with peripheral CD8/Treg ratio changes. In contrast, in mice bearing intracranial tumors, corticosteroids did not abrogate the benefits conferred by anti-PD-1 therapy. Despite systemic immune changes, anti-PD-1-mediated antitumor immune responses remained intact during corticosteroid treatment in mice bearing intracranial tumors. These findings suggest that anti-PD-1 responses may be differentially impacted by concomitant corticosteroid use depending on tumor location within or outside the CNS. As an immune-specialized site, the CNS may potentially play a protective role against the immunosuppressive effects of corticosteroids, thus sustaining antitumor immune responses mediated by PD-1 blockade.
Keywords: PD-1; brain tumor; central nervous system; colon adenocarcinoma; corticosteroid; dexamethasone; glioma; immunotherapy.
Figures


Similar articles
-
Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer.J Immunother Cancer. 2019 Sep 18;7(1):252. doi: 10.1186/s40425-019-0728-4. J Immunother Cancer. 2019. PMID: 31533840 Free PMC article.
-
Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy.J Immunother Cancer. 2018 Jun 11;6(1):51. doi: 10.1186/s40425-018-0371-5. J Immunother Cancer. 2018. PMID: 29891009 Free PMC article.
-
Combined Blockade of IL6 and PD-1/PD-L1 Signaling Abrogates Mutual Regulation of Their Immunosuppressive Effects in the Tumor Microenvironment.Cancer Res. 2018 Sep 1;78(17):5011-5022. doi: 10.1158/0008-5472.CAN-18-0118. Epub 2018 Jul 2. Cancer Res. 2018. PMID: 29967259
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Systemic CD4 Immunity as a Key Contributor to PD-L1/PD-1 Blockade Immunotherapy Efficacy.Front Immunol. 2020 Nov 30;11:586907. doi: 10.3389/fimmu.2020.586907. eCollection 2020. Front Immunol. 2020. PMID: 33329566 Free PMC article. Review.
Cited by
-
Dissociated response related to corticosteroids in lung cancer treated by immunotherapy: A case report.Clin Case Rep. 2021 Mar 24;9(5):e03973. doi: 10.1002/ccr3.3973. eCollection 2021 May. Clin Case Rep. 2021. PMID: 34026127 Free PMC article.
-
Context-Dependent Effect of Glucocorticoids on the Proliferation, Differentiation, and Apoptosis of Regulatory T Cells: A Review of the Empirical Evidence and Clinical Applications.Int J Mol Sci. 2019 Mar 6;20(5):1142. doi: 10.3390/ijms20051142. Int J Mol Sci. 2019. PMID: 30845709 Free PMC article. Review.
-
Impact of postoperative dexamethasone on survival, steroid dependency, and infections in newly diagnosed glioblastoma patients.Neurooncol Pract. 2021 Jun 23;8(5):589-600. doi: 10.1093/nop/npab039. eCollection 2021 Oct. Neurooncol Pract. 2021. PMID: 34594571 Free PMC article.
-
Inconsistencies in Modeling the Efficacy of the Oncolytic Virus HSV1716 Reveal Potential Predictive Biomarkers for Tolerability.Front Mol Biosci. 2022 Jun 15;9:889395. doi: 10.3389/fmolb.2022.889395. eCollection 2022. Front Mol Biosci. 2022. PMID: 35782876 Free PMC article.
-
Safety and efficacy of the combination of nivolumab plus ipilimumab in patients with melanoma and asymptomatic or symptomatic brain metastases (CheckMate 204).Neuro Oncol. 2021 Nov 2;23(11):1961-1973. doi: 10.1093/neuonc/noab094. Neuro Oncol. 2021. PMID: 33880555 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
