STING-dependent sensing of self-DNA drives silica-induced lung inflammation
- PMID: 30523277
- PMCID: PMC6283886
- DOI: 10.1038/s41467-018-07425-1
STING-dependent sensing of self-DNA drives silica-induced lung inflammation
Abstract
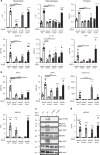
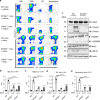
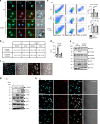
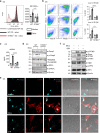
Silica particles induce lung inflammation and fibrosis. Here we show that stimulator of interferon genes (STING) is essential for silica-induced lung inflammation. In mice, silica induces lung cell death and self-dsDNA release in the bronchoalveolar space that activates STING pathway. Degradation of extracellular self-dsDNA by DNase I inhibits silica-induced STING activation and the downstream type I IFN response. Patients with silicosis have increased circulating dsDNA and CXCL10 in sputum, and patients with fibrotic interstitial lung disease display STING activation and CXCL10 in the lung. In vitro, while mitochondrial dsDNA is sensed by cGAS-STING in dendritic cells, in macrophages extracellular dsDNA activates STING independent of cGAS after silica exposure. These results reveal an essential function of STING-mediated self-dsDNA sensing after silica exposure, and identify DNase I as a potential therapy for silica-induced lung inflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Targeting STING-mediated pro-inflammatory and pro-fibrotic effects of alveolar macrophages and fibroblasts blunts silicosis caused by silica particles.J Hazard Mater. 2023 Sep 15;458:131907. doi: 10.1016/j.jhazmat.2023.131907. Epub 2023 Jun 22. J Hazard Mater. 2023. PMID: 37379600
-
Sterile Lung Inflammation Induced by Silica Exacerbates Mycobacterium tuberculosis Infection via STING-Dependent Type 2 Immunity.Cell Rep. 2019 May 28;27(9):2649-2664.e5. doi: 10.1016/j.celrep.2019.04.110. Cell Rep. 2019. PMID: 31141689
-
Self-DNA release and STING-dependent sensing drives inflammation to cigarette smoke in mice.Sci Rep. 2019 Oct 16;9(1):14848. doi: 10.1038/s41598-019-51427-y. Sci Rep. 2019. PMID: 31619733 Free PMC article.
-
Lung Inflammation in STING-Associated Vasculopathy with Onset in Infancy (SAVI).Cells. 2022 Jan 18;11(3):318. doi: 10.3390/cells11030318. Cells. 2022. PMID: 35159128 Free PMC article. Review.
-
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis.Free Radic Biol Med. 2003 Jun 15;34(12):1507-16. doi: 10.1016/s0891-5849(03)00149-7. Free Radic Biol Med. 2003. PMID: 12788471 Review.
Cited by
-
Cyclic-di-GMP Induces STING-Dependent ILC2 to ILC1 Shift During Innate Type 2 Lung Inflammation.Front Immunol. 2021 Feb 18;12:618807. doi: 10.3389/fimmu.2021.618807. eCollection 2021. Front Immunol. 2021. PMID: 33679760 Free PMC article.
-
Mitochondrial DNA Sensing Pathogen Recognition Receptors in Systemic Sclerosis Associated Interstitial Lung Disease: A Review.Curr Treatm Opt Rheumatol. 2023 Dec;9(4):204-220. doi: 10.1007/s40674-023-00211-1. Epub 2023 Aug 8. Curr Treatm Opt Rheumatol. 2023. PMID: 38230363 Free PMC article.
-
STING Targeting in Lung Diseases.Cells. 2022 Nov 3;11(21):3483. doi: 10.3390/cells11213483. Cells. 2022. PMID: 36359882 Free PMC article. Review.
-
Advancements in tyrosine kinase-mediated regulation of innate nucleic acid sensing.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 19;53(1):35-46. doi: 10.3724/zdxbyxb-2023-0480. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38426691 Free PMC article. Review. Chinese, English.
-
Inhibition of Oncogenic Src Ameliorates Silica-Induced Pulmonary Fibrosis via PI3K/AKT Pathway.Int J Mol Sci. 2023 Jan 1;24(1):774. doi: 10.3390/ijms24010774. Int J Mol Sci. 2023. PMID: 36614217 Free PMC article.
References
-
- Yoruk O, et al. The effects of silica exposure on upper airways and eyes in denim sandblasters. Rhinology. 2008;46:328–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

