Antibodies to watch in 2019
- PMID: 30516432
- PMCID: PMC6380461
- DOI: 10.1080/19420862.2018.1556465
Antibodies to watch in 2019
Abstract
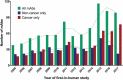
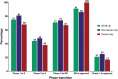
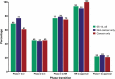
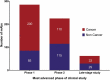
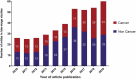
For the past 10 years, the annual 'Antibodies to watch' articles have provided updates on key events in the late-stage development of antibody therapeutics, such as first regulatory review or approval, that occurred in the year before publication or were anticipated to occur during the year of publication. To commemorate the 10th anniversary of the article series and to celebrate the 2018 Nobel Prizes in Chemistry and in Physiology or Medicine, which were given for work that is highly relevant to antibody therapeutics research and development, we expanded the scope of the data presented to include an overview of all commercial clinical development of antibody therapeutics and approval success rates for this class of molecules. Our data indicate that: 1) antibody therapeutics are entering clinical study, and being approved, in record numbers; 2) the commercial pipeline is robust, with over 570 antibody therapeutics at various clinical phases, including 62 in late-stage clinical studies; and 3) Phase 1 to approval success rates are favorable, ranging from 17-25%, depending on the therapeutic area (cancer vs. non-cancer). In 2018, a record number (12) of antibodies (erenumab (Aimovig), fremanezumab (Ajovy), galcanezumab (Emgality), burosumab (Crysvita), lanadelumab (Takhzyro), caplacizumab (Cablivi), mogamulizumab (Poteligeo), moxetumomab pasudodox (Lumoxiti), cemiplimab (Libtayo), ibalizumab (Trogarzo), tildrakizumab (Ilumetri, Ilumya), emapalumab (Gamifant)) that treat a wide variety of diseases were granted a first approval in either the European Union (EU) or United States (US). As of November 2018, 4 antibody therapeutics (sacituzumab govitecan, ravulizumab, risankizumab, romosozumab) were being considered for their first marketing approval in the EU or US, and an additional 3 antibody therapeutics developed by Chinese companies (tislelizumab, sintilimab, camrelizumab) were in regulatory review in China. In addition, our data show that 3 product candidates (leronlimab, brolucizumab, polatuzumab vedotin) may enter regulatory review by the end of 2018, and at least 12 (eptinezumab, teprotumumab, crizanlizumab, satralizumab, tanezumab, isatuximab, spartalizumab, MOR208, oportuzumab monatox, TSR-042, enfortumab vedotin, ublituximab) may enter regulatory review in 2019. Finally, we found that approximately half (18 of 33) of the late-stage pipeline of antibody therapeutics for cancer are immune checkpoint modulators or antibody-drug conjugates. Of these, 7 (tremelimumab, spartalizumab, BCD-100, omburtamab, mirvetuximab soravtansine, trastuzumab duocarmazine, and depatuxizumab mafodotin) are being evaluated in clinical studies with primary completion dates in late 2018 and in 2019, and are thus 'antibodies to watch'. We look forward to documenting progress made with these and other 'antibodies to watch' in the next installment of this article series.
Keywords: European Medicines Agency; Food and Drug Administration; antibody therapeutics; cancer; immune-mediated disorders; success rates.
Figures
Similar articles
-
Antibodies to watch in 2018.MAbs. 2018 Feb/Mar;10(2):183-203. doi: 10.1080/19420862.2018.1415671. Epub 2018 Jan 16. MAbs. 2018. PMID: 29300693 Free PMC article.
-
Antibodies to watch in 2020.MAbs. 2020 Jan-Dec;12(1):1703531. doi: 10.1080/19420862.2019.1703531. MAbs. 2020. PMID: 31847708 Free PMC article. Review.
-
Antibodies to watch in 2017.MAbs. 2017 Feb/Mar;9(2):167-181. doi: 10.1080/19420862.2016.1269580. Epub 2016 Dec 14. MAbs. 2017. PMID: 27960628 Free PMC article.
-
Antibodies to watch in 2022.MAbs. 2022 Jan-Dec;14(1):2014296. doi: 10.1080/19420862.2021.2014296. MAbs. 2022. PMID: 35030985 Free PMC article. Review.
-
Antibodies to watch in 2023.MAbs. 2023 Jan-Dec;15(1):2153410. doi: 10.1080/19420862.2022.2153410. MAbs. 2023. PMID: 36472472 Free PMC article.
Cited by
-
The Contorsbody, an antibody format for agonism: Design, structure, and function.Comput Struct Biotechnol J. 2020 May 14;18:1210-1220. doi: 10.1016/j.csbj.2020.05.007. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32542107 Free PMC article.
-
Process Analytical Technologies and Data Analytics for the Manufacture of Monoclonal Antibodies.Trends Biotechnol. 2020 Oct;38(10):1169-1186. doi: 10.1016/j.tibtech.2020.07.004. Epub 2020 Aug 21. Trends Biotechnol. 2020. PMID: 32839030 Free PMC article. Review.
-
On-column disulfide bond formation of monoclonal antibodies during Protein A chromatography eliminates low molecular weight species and rescues reduced antibodies.MAbs. 2020 Jan-Dec;12(1):1829333. doi: 10.1080/19420862.2020.1829333. MAbs. 2020. PMID: 33016217 Free PMC article.
-
Advances in the Production and Batch Reformatting of Phage Antibody Libraries.Mol Biotechnol. 2019 Nov;61(11):801-815. doi: 10.1007/s12033-019-00207-0. Mol Biotechnol. 2019. PMID: 31468301 Free PMC article. Review.
-
Thera-SAbDab: the Therapeutic Structural Antibody Database.Nucleic Acids Res. 2020 Jan 8;48(D1):D383-D388. doi: 10.1093/nar/gkz827. Nucleic Acids Res. 2020. PMID: 31555805 Free PMC article.
References
-
- The Royal Swedish Academy of Sciences Scientific background on the Nobel Prize in Chemistry 2018: directed evolution of enzymes and binding proteins. https://www.nobelprize.org/uploads/2018/10/advanced-chemistryprize-2018.pdf
-
- Scott JK, Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249(4967):386–390. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
