Targeted migration of bone marrow mesenchymal stem cells inhibits silica-induced pulmonary fibrosis in rats
- PMID: 30514375
- PMCID: PMC6280342
- DOI: 10.1186/s13287-018-1083-y
Targeted migration of bone marrow mesenchymal stem cells inhibits silica-induced pulmonary fibrosis in rats
Abstract
Background: Silicosis is a common occupational disease, characterized by silicotic nodules and diffuse pulmonary fibrosis. We demonstrated an anti-fibrotic effect of bone marrow mesenchymal stem cells (BMSCs) in silica-induced lung fibrosis. In the present study, we sought to clarify the homing ability of BMSCs and the specific mechanisms for their effects.
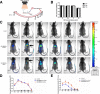
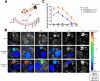
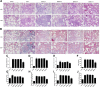
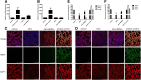
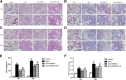
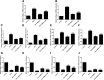
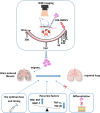
Methods and results: The biodistribution of BMSCs was identified by near-infrared fluorescence (NIRF) imaging in vivo and in vitro. The results showed that BMSCs labeled with NIR-DiR dyes targeted silica-injured lung tissue, wherein they reached a peak at 6 h post-injection and declined dramatically by day 3. Based on these findings, a second injection of BMSCs was administered 3 days after the first injection. The injected BMSCs migrated to the injured lungs, but did not undergo transformation into specific lung cell types. Interestingly, the injection of BMSC-conditioned medium (BMSCs-CM) significantly attenuated silica-induced pulmonary fibrosis. The collagen deposition and number of nodules were decreased in lung tissues of BMSCs-CM-treated rats. In parallel with these findings, the mRNA levels of collagen I, collagen III, and fibronectin, and the content of transforming growth factor (TGF)-β1 and hydroxyproline were decreased in the BMSCs-CM-treated group compared with the silica group. In addition, alveolar epithelial markers were upregulated by BMSCs-CM treatment.
Conclusions: BMSCs migrated to injured areas of the lung after silica instillation and attenuated pulmonary fibrosis. The anti-fibrotic effects of BMSCs were mainly exerted in paracrine manner, rather than through their ability to undergo differentiation.
Keywords: Bone marrow mesenchymal stem cells; Migration; Pulmonary fibrosis; Silicosis.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in the study involving animals were obeyed by the ethical standards of the institution detailed in “Materials and methods”.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/β-catenin signaling in rats.Stem Cell Res Ther. 2018 Nov 14;9(1):311. doi: 10.1186/s13287-018-1045-4. Stem Cell Res Ther. 2018. PMID: 30428918 Free PMC article.
-
Anti-fibrotic effects of bone morphogenetic protein-7-modified bone marrow mesenchymal stem cells on silica-induced pulmonary fibrosis.Exp Mol Pathol. 2017 Feb;102(1):70-77. doi: 10.1016/j.yexmp.2016.12.010. Epub 2017 Jan 4. Exp Mol Pathol. 2017. PMID: 28062213
-
Bone marrow mesenchymal stem cells attenuate silica-induced pulmonary fibrosis via paracrine mechanisms.Toxicol Lett. 2017 Mar 15;270:96-107. doi: 10.1016/j.toxlet.2017.02.016. Epub 2017 Feb 21. Toxicol Lett. 2017. PMID: 28232222
-
A Mini-Review: The Therapeutic Potential of Bone Marrow Mesenchymal Stem Cells and Relevant Signaling Cascades.Curr Stem Cell Res Ther. 2019;14(3):214-218. doi: 10.2174/1574888X13666180912141228. Curr Stem Cell Res Ther. 2019. PMID: 30207242 Review.
-
[Application of mesenchymal stem cells to liver regenerative medicine].Yakugaku Zasshi. 2008 Jan;128(1):3-9. doi: 10.1248/yakushi.128.3. Yakugaku Zasshi. 2008. PMID: 18176050 Review. Japanese.
Cited by
-
Mesenchymal Stem/Stromal Cells in Progressive Fibrogenic Involvement and Anti-Fibrosis Therapeutic Properties.Front Cell Dev Biol. 2022 Jun 1;10:902677. doi: 10.3389/fcell.2022.902677. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35721482 Free PMC article. Review.
-
Cellular Therapy for the Treatment of Paediatric Respiratory Disease.Int J Mol Sci. 2021 Aug 18;22(16):8906. doi: 10.3390/ijms22168906. Int J Mol Sci. 2021. PMID: 34445609 Free PMC article. Review.
-
The role of pyroptosis in inflammatory diseases.Front Cell Dev Biol. 2023 May 12;11:1173235. doi: 10.3389/fcell.2023.1173235. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37250902 Free PMC article. Review.
-
Herbal compounds in the treatment of pulmonary silicosis.Physiol Res. 2021 Dec 31;70(S3):S275-S287. doi: 10.33549/physiolres.934817. Physiol Res. 2021. PMID: 35099247 Free PMC article. Review.
-
Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial.Stem Cell Res Ther. 2022 Mar 21;13(1):122. doi: 10.1186/s13287-022-02796-1. Stem Cell Res Ther. 2022. PMID: 35313959 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

