Ombitasvir/paritaprevir/ritonavir+dasabuvir and ribavirin associated drug-induced liver injury and syndrome of inappropriate secretion of anti-diuretic hormone: A case report
- PMID: 30509013
- PMCID: PMC6759438
- DOI: 10.3350/cmh.2018.0063
Ombitasvir/paritaprevir/ritonavir+dasabuvir and ribavirin associated drug-induced liver injury and syndrome of inappropriate secretion of anti-diuretic hormone: A case report
Conflict of interest statement
The authors have no conflicts to disclose.
Figures
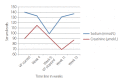
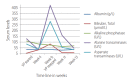
Similar articles
-
Efficacy and Safety of Ombitasvir/Paritaprevir/ Ritonavir + Dasabuvir ± Ribavirin Combinations in Patients with Genotype 1 Hepatitis C and Inherited Bleeding Disorders.Turk J Gastroenterol. 2022 May;33(5):414-420. doi: 10.5152/tjg.2022.20844. Turk J Gastroenterol. 2022. PMID: 35678799 Free PMC article.
-
Profile of paritaprevir/ritonavir/ombitasvir plus dasabuvir in the treatment of chronic hepatitis C virus genotype 1 infection.Drug Des Devel Ther. 2015 Nov 13;9:6083-94. doi: 10.2147/DDDT.S80226. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26622169 Free PMC article. Review.
-
Efficacy and safety of ombitasvir/paritaprevir/ritonavir and dasabuvir with low-dose ribavirin in patients with chronic hepatitis C virus genotype 1a infection without cirrhosis.J Viral Hepat. 2019 Aug;26(8):1027-1030. doi: 10.1111/jvh.13109. Epub 2019 May 6. J Viral Hepat. 2019. PMID: 30980576 Free PMC article. Clinical Trial.
-
Ombitasvir/Paritaprevir/Ritonavir Plus Dasabuvir: A Review in Chronic HCV Genotype 1 Infection.Drugs. 2015 Jun;75(9):1027-38. doi: 10.1007/s40265-015-0412-z. Drugs. 2015. PMID: 26059288 Review.
-
Ombitasvir/paritaprevir/ritonavir and dasabuvir tablets for hepatitis C virus genotype 1 infection.Ann Pharmacother. 2015 May;49(5):566-81. doi: 10.1177/1060028015570729. Epub 2015 Feb 13. Ann Pharmacother. 2015. PMID: 25680759 Review.
Cited by
-
HCV Antiviral Drugs Have the Potential to Adversely Perturb the Fetal-Maternal Communication Axis through Inhibition of CYP3A7 DHEA-S Oxidation.Drug Metab Dispos. 2024 May 16;52(6):516-525. doi: 10.1124/dmd.123.001434. Drug Metab Dispos. 2024. PMID: 38267095 Free PMC article.
-
Arginine vasopressin and pathophysiology of COVID-19: An innovative perspective.Biomed Pharmacother. 2021 Nov;143:112193. doi: 10.1016/j.biopha.2021.112193. Epub 2021 Sep 15. Biomed Pharmacother. 2021. PMID: 34543987 Free PMC article. Review.
References
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet. 2001;358:958–965. - PubMed
-
- Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. - PubMed
-
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-460/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982. - PubMed
-
- American Associations for the Study of Liver Diseases (AASLD) HCV guidance: recommendations for testing, managing, and treating hepatitis C. AASLD web site, < https://www.hcvguidelines.org/treatment-naive/gt1>. Accessed 15 Jun 2018. - PubMed
-
- European Association for the Study of the Liver (EASL) EASL recommendations on treatment of hepatitis C 2016 Summary. EASL web site, < http://www.easl.eu/medias/cpg/HCV2016/Summary.pdf>. Accessed 15 Jun 2018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

