Restriction of Human Cytomegalovirus Infection by Galectin-9
- PMID: 30487283
- PMCID: PMC6340044
- DOI: 10.1128/JVI.01746-18
Restriction of Human Cytomegalovirus Infection by Galectin-9
Abstract
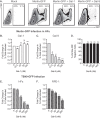
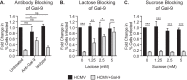
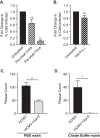
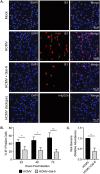
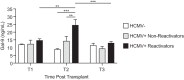
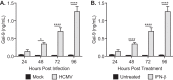
Human cytomegalovirus (HCMV) is a ubiquitous human herpesvirus. While HCMV infection is generally asymptomatic in the immunocompetent, it can have devastating consequences in those with compromised or underdeveloped immune systems, including transplant recipients and neonates. Galectins are a widely expressed protein family that have been demonstrated to modulate both antiviral immunity and regulate direct host-virus interactions. The potential for galectins to directly modulate HCMV infection has not previously been studied, and our results reveal that galectin-9 (Gal-9) can potently inhibit HCMV infection. Gal-9-mediated inhibition of HCMV was dependent upon its carbohydrate recognition domains and thus dependent on glycan interactions. Temperature shift studies revealed that Gal-9 specific inhibition was mediated primarily at the level of virus-cell fusion and not binding. Additionally, we found that during reactivation of HCMV in hematopoietic stem cell transplant (HSCT) patients soluble Gal-9 is upregulated. This study provides the first evidence for Gal-9 functioning as a potent antiviral defense effector molecule against HCMV infection and identifies it as a potential clinical candidate to restrict HCMV infections.IMPORTANCE Human cytomegalovirus (HCMV) continues to cause serious and often life-threatening disease in those with impaired or underdeveloped immune systems. This virus is able to infect and replicate in a wide range of human cell types, which enables the virus to spread to other individuals in a number of settings. Current antiviral drugs are associated with a significant toxicity profile, and there is no vaccine; these factors highlight a need to identify additional targets for the development of anti-HCMV therapies. We demonstrate for the first time that secretion of a member of the galectin family of proteins, galectin-9 (Gal-9), is upregulated during natural HCMV-reactivated infection and that this soluble cellular protein possesses a potent capacity to block HCMV infection by inhibiting virus entry into the host cell. Our findings support the possibility of harnessing the antiviral properties of Gal-9 to prevent HCMV infection and disease.
Keywords: cytomegalovirus; human herpesviruses; virus-host interactions.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Human cytomegalovirus upregulates expression of the lectin galectin 9 via induction of beta interferon.J Virol. 2014 Sep;88(18):10990-4. doi: 10.1128/JVI.01259-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008927 Free PMC article.
-
Anticytomegalovirus Peptides Point to New Insights for CMV Entry Mechanisms and the Limitations of In Vitro Screenings.mSphere. 2019 Feb 13;4(1):e00586-18. doi: 10.1128/mSphere.00586-18. mSphere. 2019. PMID: 30760613 Free PMC article.
-
Expression of Oncogenic Alleles Induces Multiple Blocks to Human Cytomegalovirus Infection.J Virol. 2016 Apr 14;90(9):4346-4356. doi: 10.1128/JVI.00179-16. Print 2016 May. J Virol. 2016. PMID: 26889030 Free PMC article.
-
Cytomegalovirus and varicella-zoster virus vaccines in hematopoietic stem cell transplantation.Expert Rev Vaccines. 2009 Aug;8(8):999-1021. doi: 10.1586/erv.09.58. Expert Rev Vaccines. 2009. PMID: 19627184 Review.
-
[Interrelationship between human cytomegalovirus infection and chemokine].Nihon Rinsho. 1998 Jan;56(1):69-74. Nihon Rinsho. 1998. PMID: 9465667 Review. Japanese.
Cited by
-
Blood Levels of Galectin-9, an Immuno-Regulating Molecule, Reflect the Severity for the Acute and Chronic Infectious Diseases.Biomolecules. 2021 Mar 15;11(3):430. doi: 10.3390/biom11030430. Biomolecules. 2021. PMID: 33804076 Free PMC article. Review.
-
Human galectin-9 potently enhances SARS-CoV-2 replication and inflammation in airway epithelial cells.J Mol Cell Biol. 2023 Aug 3;15(4):mjad030. doi: 10.1093/jmcb/mjad030. J Mol Cell Biol. 2023. PMID: 37127426 Free PMC article.
-
Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection.mBio. 2021 May 4;12(3):e00384-21. doi: 10.1128/mBio.00384-21. mBio. 2021. PMID: 33947753 Free PMC article.
-
Tuning the Orchestra: HCMV vs. Innate Immunity.Front Microbiol. 2020 Apr 15;11:661. doi: 10.3389/fmicb.2020.00661. eCollection 2020. Front Microbiol. 2020. PMID: 32351486 Free PMC article. Review.
-
Intrinsic Immune Mechanisms Restricting Human Cytomegalovirus Replication.Viruses. 2021 Jan 26;13(2):179. doi: 10.3390/v13020179. Viruses. 2021. PMID: 33530304 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

