FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells
- PMID: 30486505
- PMCID: PMC6321370
- DOI: 10.3390/molecules23123110
FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells
Abstract
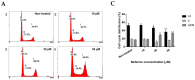
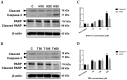
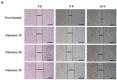
Human neuroblastoma cancer is the most typical extracranial solid tumor. Yet, new remedial treatment therapies are demanded to overcome its sluggish survival rate. Neferine, isolated from the lotus embryos, inhibits the proliferation of various cancer cells. This study aimed to evaluate the anti-cancer activity of neferine in IMR32 human neuroblastoma cells and to expose the concealable molecular mechanisms. IMR32 cells were treated with different concentrations of neferine, followed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to assess cell viability. In an effort to determine the molecular mechanisms in neferine-incubated IMR32 cells, cell cycle arrest, cell migration, and focal adhesion kinase (FAK), the 70-kDa ribosomal S6 kinase 1 (S6K1), poly (ADP-ribose) polymerase (PARP), caspase-3, Beclin-1, and microtubule-associated protein 1A/1B-light chain 3 (LC3) protein expressions were investigated. Neferine strongly disrupted the neuroblastoma cell growth via induction of G2/M phase arrest. Furthermore, neferine provoked autophagy and apoptosis in IMR32 cells, confirmed by p-FAK, and p-S6K1 reduction, LC3-II accumulation, Beclin-1 overexpression, and cleaved caspase-3/PARP improvement. Finally, neferine markedly retarded cell migration of neuroblastoma cancer cells. As a result, our findings for the first time showed an explicit anti-cancer effect of neferine in IMR32 cells, suggesting that neferine might be a potential candidate against human neuroblastoma cells to improve clinical outcomes with further in vivo investigation.
Keywords: FAK/S6K1; apoptosis; autophagy; human neuroblastoma cells; neferine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Neferine isolated from Nelumbo nucifera enhances anti-cancer activities in Hep3B cells: molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response.Phytomedicine. 2013 Aug 15;20(11):1013-22. doi: 10.1016/j.phymed.2013.03.024. Epub 2013 Jun 6. Phytomedicine. 2013. PMID: 23746959
-
Tetrandrine induces cell death in SAS human oral cancer cells through caspase activation-dependent apoptosis and LC3-I and LC3-II activation-dependent autophagy.Int J Oncol. 2013 Aug;43(2):485-94. doi: 10.3892/ijo.2013.1952. Epub 2013 May 21. Int J Oncol. 2013. PMID: 23695424
-
Neferine Potentiates the Antitumor Effect of Cisplatin in Human Lung Adenocarcinoma Cells Via a Mitochondria-Mediated Apoptosis Pathway.J Cell Biochem. 2017 Sep;118(9):2865-2876. doi: 10.1002/jcb.25937. Epub 2017 Apr 27. J Cell Biochem. 2017. PMID: 28214344
-
Pharmacological benefits of neferine - A comprehensive review.Life Sci. 2018 Apr 15;199:60-70. doi: 10.1016/j.lfs.2018.02.032. Epub 2018 Feb 27. Life Sci. 2018. PMID: 29499283 Review.
-
The Cytoprotective and Anti-cancer Potential of Bisbenzylisoquinoline Alkaloids from Nelumbo nucifera.Curr Top Med Chem. 2019;19(32):2940-2957. doi: 10.2174/1568026619666191116160908. Curr Top Med Chem. 2019. PMID: 31738137 Review.
Cited by
-
Potential Focal Adhesion Kinase Inhibitors in Management of Cancer: Therapeutic Opportunities from Herbal Medicine.Int J Mol Sci. 2022 Nov 1;23(21):13334. doi: 10.3390/ijms232113334. Int J Mol Sci. 2022. PMID: 36362132 Free PMC article. Review.
-
Targeting Autophagy with Natural Products as a Potential Therapeutic Approach for Cancer.Int J Mol Sci. 2021 Sep 10;22(18):9807. doi: 10.3390/ijms22189807. Int J Mol Sci. 2021. PMID: 34575981 Free PMC article. Review.
-
Neferine induces p38 MAPK/JNK1/2 activation to modulate melanoma proliferation, apoptosis, and oxidative stress.Ann Transl Med. 2020 Dec;8(24):1643. doi: 10.21037/atm-20-7201. Ann Transl Med. 2020. PMID: 33490155 Free PMC article.
-
Antitumoral Properties of Natural Products.Molecules. 2020 Feb 3;25(3):650. doi: 10.3390/molecules25030650. Molecules. 2020. PMID: 32028725 Free PMC article.
-
Neferine induces apoptosis by modulating the ROS‑mediated JNK pathway in esophageal squamous cell carcinoma.Oncol Rep. 2020 Sep;44(3):1116-1126. doi: 10.3892/or.2020.7675. Epub 2020 Jul 7. Oncol Rep. 2020. PMID: 32705225 Free PMC article.
References
-
- Matthay K.K., Villablanca J.G., Seeger R.C., Stram D.O., Harris R.E., Ramsay N.K., Swift P., Shimada H., Black C.T., Brodeur G.M., et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. New Engl. J. Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

