Selective agonist of TRPML2 reveals direct role in chemokine release from innate immune cells
- PMID: 30479274
- PMCID: PMC6257821
- DOI: 10.7554/eLife.39720
Selective agonist of TRPML2 reveals direct role in chemokine release from innate immune cells
Abstract
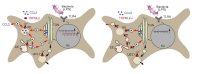
Cytokines and chemokines are produced and secreted by a broad range of immune cells including macrophages. Remarkably, little is known about how these inflammatory mediators are released from the various immune cells. Here, the endolysosomal cation channel TRPML2 is shown to play a direct role in chemokine trafficking and secretion from murine macrophages. To demonstrate acute and direct involvement of TRPML2 in these processes, the first isoform-selective TRPML2 channel agonist was generated, ML2-SA1. ML2-SA1 was not only found to directly stimulate release of the chemokine CCL2 from macrophages but also to stimulate macrophage migration, thus mimicking CCL2 function. Endogenous TRPML2 is expressed in early/recycling endosomes as demonstrated by endolysosomal patch-clamp experimentation and ML2-SA1 promotes trafficking through early/recycling endosomes, suggesting CCL2 being transported and secreted via this pathway. These data provide a direct link between TRPML2 activation, CCL2 release and stimulation of macrophage migration in the innate immune response.
Keywords: CCL2; Mcoln2; TRPML; TRPML2; biochemistry; chemical biology; lysosome; macrophage; mouse.
© 2018, Plesch et al.
Conflict of interest statement
EP, CC, EB, AS, EK, HY, KB, MK, DR, DT, LH, AV, WS, RP, DM, MB, CW, FB, CG No competing interests declared
Figures
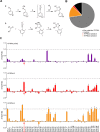
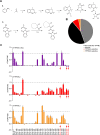



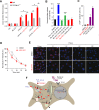
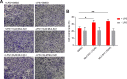
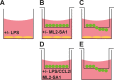
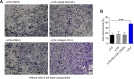
Comment in
-
Revealing the secrets of secretion.Elife. 2018 Nov 30;7:e43512. doi: 10.7554/eLife.43512. Elife. 2018. PMID: 30499445 Free PMC article.
Similar articles
-
Functional In Vitro Assessment of VEGFA/NOTCH2 Signaling Pathway and pRB Proteasomal Degradation and the Clinical Relevance of Mucolipin TRPML2 Overexpression in Glioblastoma Patients.Int J Mol Sci. 2022 Jan 8;23(2):688. doi: 10.3390/ijms23020688. Int J Mol Sci. 2022. PMID: 35054871 Free PMC article.
-
Novel Role of TRPML2 in the Regulation of the Innate Immune Response.J Immunol. 2015 Nov 15;195(10):4922-32. doi: 10.4049/jimmunol.1500163. Epub 2015 Oct 2. J Immunol. 2015. PMID: 26432893 Free PMC article.
-
ML-SA1 and SN-2 inhibit endocytosed viruses through regulating TRPML channel expression and activity.Antiviral Res. 2021 Nov;195:105193. doi: 10.1016/j.antiviral.2021.105193. Epub 2021 Oct 20. Antiviral Res. 2021. PMID: 34687820
-
TRPML Cation Channels in Inflammation and Immunity.Front Immunol. 2020 Feb 28;11:225. doi: 10.3389/fimmu.2020.00225. eCollection 2020. Front Immunol. 2020. PMID: 32184778 Free PMC article. Review.
-
Two-pore and TRP cation channels in endolysosomal osmo-/mechanosensation and volume regulation.Biochim Biophys Acta Mol Cell Res. 2021 Feb;1868(2):118921. doi: 10.1016/j.bbamcr.2020.118921. Epub 2020 Dec 3. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33279607 Review.
Cited by
-
Non-viral approaches in CAR-NK cell engineering: connecting natural killer cell biology and gene delivery.J Nanobiotechnology. 2024 Sep 10;22(1):552. doi: 10.1186/s12951-024-02746-4. J Nanobiotechnology. 2024. PMID: 39256765 Free PMC article. Review.
-
Lysosomal TRPML1 regulates mitochondrial function in hepatocellular carcinoma cells.J Cell Sci. 2022 Mar 15;135(6):jcs259455. doi: 10.1242/jcs.259455. Epub 2022 Mar 21. J Cell Sci. 2022. PMID: 35274126 Free PMC article.
-
Endolysosomal Cation Channels and Lung Disease.Cells. 2022 Jan 17;11(2):304. doi: 10.3390/cells11020304. Cells. 2022. PMID: 35053420 Free PMC article. Review.
-
Endolysosomal transient receptor potential mucolipins and two-pore channels: implications for cancer immunity.Front Immunol. 2024 May 22;15:1389194. doi: 10.3389/fimmu.2024.1389194. eCollection 2024. Front Immunol. 2024. PMID: 38840905 Free PMC article. Review.
-
Functional In Vitro Assessment of VEGFA/NOTCH2 Signaling Pathway and pRB Proteasomal Degradation and the Clinical Relevance of Mucolipin TRPML2 Overexpression in Glioblastoma Patients.Int J Mol Sci. 2022 Jan 8;23(2):688. doi: 10.3390/ijms23020688. Int J Mol Sci. 2022. PMID: 35054871 Free PMC article.
References
-
- Akiba K, Kashiwagi K, Ohyama Y, Yamamoto Y, Ohkata K. Ring transformation equilibrium (bond switch) in 5-(2-aminovinyl)isothiazole system via hypervalent sulfurane. synthesis, structure determination, and kinetic study. Journal of the American Chemical Society. 1985;107:2721–2730. doi: 10.1021/ja00295a026. - DOI
-
- Bala S, Saini M, Kamboj S, Saini V. Synthesis Of 2-[4-(Substituted Benzylidene)-5-Oxo-4,5-Dihydro-Oxazol-2-Ylmethyl]-Isoindole-1,3-Dione derivatives as novel potential antimicrobial agents. Iranian Journal of Pharmacology and Therapeutics. 2012;2012:45–52.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

