Characterizing the Different Effects of Zika Virus Infection in Placenta and Microglia Cells
- PMID: 30453684
- PMCID: PMC6266000
- DOI: 10.3390/v10110649
Characterizing the Different Effects of Zika Virus Infection in Placenta and Microglia Cells
Abstract
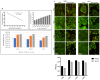
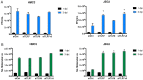
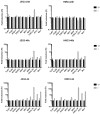
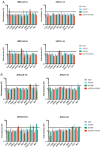
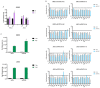
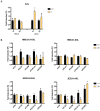
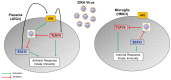
Zika virus (ZIKV) is a neuropathic virus that causes serious neurological abnormalities such as Guillain-Barre syndrome in adults and congenital Zika syndrome (CZS) in fetuses, which makes it an important concern for global human health. A catalogue of cells that support ZIKV replication, pathogenesis, and/or the persistence of the virus still remains unknown. Here, we studied the behavior of the virus in human placenta (JEG-3) and human microglia (HMC3) cell lines in order to better understand how different host tissues respond during infection. We quantified the host transcriptional response to ZIKV infection in both types of cells at 24 and 72 h post-infection. A panel of 84 genes that are involved in the innate or adaptive immune responses was used to quantify differential expression in both cell lines. HMC3 cells showed a unique set of significant differentially expressed genes (DEGs) compared with JEG-3 cells at both time points. Subsequent analysis of these data using modern pathway analysis methods revealed that the TLR7/8 pathway was strongly inhibited in HMC3 cells, while it was activated in JEG-3 cells during virus infection. The disruption of these pathways was subsequently confirmed with specific small interfering RNA (siRNA) experiments that characterize their role in the viral life cycle, and may partially explain why ZIKV infection in placental tissue contributes to extreme neurological problems in a developing fetus.
Keywords: TLR7/8; microglia cells; placenta cells; siRNA; zika virus.
Conflict of interest statement
The authors declare no conflict of interest
Figures
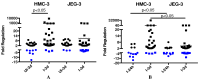
Similar articles
-
Zika virus infection reprograms global transcription of host cells to allow sustained infection.Emerg Microbes Infect. 2017 Apr 26;6(4):e24. doi: 10.1038/emi.2017.9. Emerg Microbes Infect. 2017. PMID: 28442752 Free PMC article.
-
A CRISPR Activation Screen Identifies Genes That Protect against Zika Virus Infection.J Virol. 2019 Jul 30;93(16):e00211-19. doi: 10.1128/JVI.00211-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142663 Free PMC article.
-
Hydroxycarboxylic Acid Receptor 2 Is a Zika Virus Restriction Factor That Can Be Induced by Zika Virus Infection Through the IRE1-XBP1 Pathway.Front Cell Infect Microbiol. 2020 Jan 22;9:480. doi: 10.3389/fcimb.2019.00480. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 32039055 Free PMC article.
-
Congenital Zika syndrome: Pitfalls in the placental barrier.Rev Med Virol. 2018 Sep;28(5):e1985. doi: 10.1002/rmv.1985. Epub 2018 May 15. Rev Med Virol. 2018. PMID: 29761581 Review.
-
Zika virus: Molecular responses and tissue tropism in the mammalian host.Rev Med Virol. 2019 Jul;29(4):e2050. doi: 10.1002/rmv.2050. Epub 2019 May 16. Rev Med Virol. 2019. PMID: 31095819 Review.
Cited by
-
Absence of Zika virus among pregnant women in Vietnam in 2008.Trop Dis Travel Med Vaccines. 2023 Mar 1;9(1):4. doi: 10.1186/s40794-023-00189-7. Trop Dis Travel Med Vaccines. 2023. PMID: 36855197 Free PMC article.
-
High-Throughput SARS-CoV-2 Antiviral Testing Method Using the Celigo Image Cytometer.J Fluoresc. 2024 Mar;34(2):561-570. doi: 10.1007/s10895-023-03289-x. Epub 2023 Jun 13. J Fluoresc. 2024. PMID: 37310590 Free PMC article.
-
New Advances on Zika Virus Research.Viruses. 2019 Mar 14;11(3):258. doi: 10.3390/v11030258. Viruses. 2019. PMID: 30875715 Free PMC article.
-
Zika virus infection induces endoplasmic reticulum stress and apoptosis in placental trophoblasts.Cell Death Discov. 2021 Jan 26;7(1):24. doi: 10.1038/s41420-020-00379-8. Cell Death Discov. 2021. PMID: 33500388 Free PMC article.
-
Zika Virus Neuropathogenesis: The Different Brain Cells, Host Factors and Mechanisms Involved.Front Immunol. 2022 Mar 16;13:773191. doi: 10.3389/fimmu.2022.773191. eCollection 2022. Front Immunol. 2022. PMID: 35371036 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

