Soluble Urokinase Plasminogen Activator Receptor Is Predictive of Non-AIDS Events During Antiretroviral Therapy-mediated Viral Suppression
- PMID: 30418519
- PMCID: PMC6669298
- DOI: 10.1093/cid/ciy966
Soluble Urokinase Plasminogen Activator Receptor Is Predictive of Non-AIDS Events During Antiretroviral Therapy-mediated Viral Suppression
Abstract
Background: Despite effective antiretroviral therapy (ART), human immunodeficiency virus (HIV) infection remains associated with higher morbidity and mortality, driven, in part, by increased inflammation. Our objective was to identify associations between levels of plasma biomarkers of chronic inflammation, microbial translocation, and monocyte activation, with occurrence of non-AIDS events.
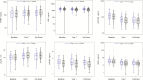
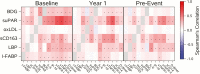
Methods: Participants (141 cases, 310 matched controls) were selected from a longitudinal observational trial; all were virally suppressed on ART at year 1 and thereafter. Soluble urokinase plasminogen activator receptor (suPAR), lipopolysaccharide binding protein (LBP), beta-D-glucan (BDG), intestinal fatty-acid binding protein, oxidized low-density lipoproteins, and soluble CD163 were measured pre-ART, after 1-year of ART, and pre-event. At each time point, conditional logistic regression analysis assessed associations of the biomarkers with events and adjusted for relevant covariates to calculate odds ratios (ORs) according to 1 interquartile range (IQR) difference.
Results: At all time points, higher levels of suPAR were associated with increased risk of non-AIDS events (OR per 1 IQR was 1.7 before ART-initiation, OR per 1 IQR was 2.0 after 1 year of suppressive ART, and OR 2.1 pre-event). Higher levels of BDG and LBP at year 1 and pre-event (but not at baseline) were associated with increased risk of non-AIDS events. No associations were observed for other biomarkers.
Conclusions: Elevated levels of suPAR were strongly, consistently, and independently predictive of non-AIDS events at every measured time point. Interventions that target the suPAR pathway should be investigated to explore its role in the pathogenesis of non-AIDS-related outcomes in HIV infection.
Keywords: beta-D-glucan; lipopolysaccharide binding protein; non-AIDS mortality; suPAR; viral suppression.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Plasma (1 → 3)-β-D-glucan and suPAR levels correlate with neurocognitive performance in people living with HIV on antiretroviral therapy: a CHARTER analysis.J Neurovirol. 2019 Dec;25(6):837-843. doi: 10.1007/s13365-019-00775-6. Epub 2019 Jul 11. J Neurovirol. 2019. PMID: 31297727 Free PMC article.
-
Soluble urokinase plasminogen activator receptor (suPAR) is a novel, independent predictive marker of myocardial infarction in HIV-1-infected patients: a nested case-control study.HIV Med. 2016 May;17(5):350-7. doi: 10.1111/hiv.12315. Epub 2015 Sep 14. HIV Med. 2016. PMID: 26365671 Free PMC article.
-
Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment.J Infect Dis. 2014 Oct 15;210(8):1248-59. doi: 10.1093/infdis/jiu254. Epub 2014 May 1. J Infect Dis. 2014. PMID: 24795473 Free PMC article.
-
Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection.Clin Infect Dis. 2017 Jan 15;64(2):124-131. doi: 10.1093/cid/ciw683. Epub 2016 Oct 12. Clin Infect Dis. 2017. PMID: 27737952 Free PMC article.
-
Relationship between serum soluble urokinase plasminogen activator receptor level and steroid responsiveness in FSGS.Clin J Am Soc Nephrol. 2014 Nov 7;9(11):1903-11. doi: 10.2215/CJN.02370314. Epub 2014 Oct 15. Clin J Am Soc Nephrol. 2014. PMID: 25318750 Free PMC article.
Cited by
-
Glucan rich nutrition does not increase gut translocation of beta-glucan.Mycoses. 2021 Jan;64(1):24-29. doi: 10.1111/myc.13161. Epub 2020 Nov 21. Mycoses. 2021. PMID: 32780885 Free PMC article.
-
Invasive aspergillosis in critically ill patients: Review of definitions and diagnostic approaches.Mycoses. 2021 Sep;64(9):1002-1014. doi: 10.1111/myc.13274. Epub 2021 Apr 6. Mycoses. 2021. PMID: 33760284 Free PMC article. Review.
-
Fungal Translocation: A Driving Force Behind the Occurrence of Non-AIDS Events?Clin Infect Dis. 2020 Jan 2;70(2):242-244. doi: 10.1093/cid/ciz215. Clin Infect Dis. 2020. PMID: 30861074 Free PMC article. No abstract available.
-
Changes in the Fungal Marker β-D-Glucan After Antiretroviral Therapy and Association With Adiposity.Open Forum Infect Dis. 2019 Nov 11;6(11):ofz434. doi: 10.1093/ofid/ofz434. eCollection 2019 Nov. Open Forum Infect Dis. 2019. PMID: 31737737 Free PMC article.
-
Cytomegalovirus Seropositivity Is Associated With Increased Microbial Translocation in People Living With Human Immunodeficiency Virus and Uninfected Controls.Clin Infect Dis. 2020 Sep 12;71(6):1438-1446. doi: 10.1093/cid/ciz1001. Clin Infect Dis. 2020. PMID: 31608409 Free PMC article.
References
-
- Palella FJ Jr , Baker RK , Moorman AC , et al. ; HIV Outpatient Study Investigators Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. - PubMed
-
- Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

