Interleukin-17 Drives Interstitial Entrapment of Tissue Lipoproteins in Experimental Psoriasis
- PMID: 30415924
- PMCID: PMC6365189
- DOI: 10.1016/j.cmet.2018.10.006
Interleukin-17 Drives Interstitial Entrapment of Tissue Lipoproteins in Experimental Psoriasis
Abstract
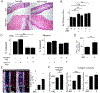
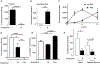
Lipoproteins trapped in arteries drive atherosclerosis. Extravascular low-density lipoprotein undergoes receptor uptake, whereas high-density lipoprotein (HDL) interacts with cells to acquire cholesterol and then recirculates to plasma. We developed photoactivatable apoA-I to understand how HDL passage through tissue is regulated. We focused on skin and arteries of healthy mice versus those with psoriasis, which carries cardiovascular risk in man. Our findings suggest that psoriasis-affected skin lesions program interleukin-17-producing T cells in draining lymph nodes to home to distal skin and later to arteries. There, these cells mediate thickening of the collagenous matrix, such that larger molecules including lipoproteins become entrapped. HDL transit was rescued by depleting CD4+ T cells, neutralizing interleukin-17, or inhibiting lysyl oxidase that crosslinks collagen. Experimental psoriasis also increased vascular stiffness and atherosclerosis via this common pathway. Thus, interleukin-17 can reduce lipoprotein trafficking and increase vascular stiffness by, at least in part, remodeling collagen.
Keywords: Th17 immunity; artery; atherosclerosis; autoimmunity; collagen; cytokines; extracellular matrix; fibrosis; interstitial transport; skin.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no conflicts of interest.
Figures
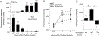
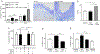

Similar articles
-
Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation.Circ Res. 2005 Oct 14;97(8):763-71. doi: 10.1161/01.RES.0000185320.82962.F7. Epub 2005 Sep 8. Circ Res. 2005. PMID: 16151025
-
Matrix metalloproteinase 8 degrades apolipoprotein A-I and reduces its cholesterol efflux capacity.FASEB J. 2015 Apr;29(4):1435-45. doi: 10.1096/fj.14-262956. Epub 2014 Dec 30. FASEB J. 2015. PMID: 25550459
-
Multi-glycoside of Tripterygium wilfordii Hook. f. ameliorates imiquimod-induced skin lesions through a STAT3-dependent mechanism involving the inhibition of Th17-mediated inflammatory responses.Int J Mol Med. 2016 Sep;38(3):747-57. doi: 10.3892/ijmm.2016.2670. Epub 2016 Jul 11. Int J Mol Med. 2016. PMID: 27431437 Free PMC article.
-
Antipsoriatic treatment extends beyond the skin: recovering of high-density lipoprotein function.Exp Dermatol. 2014 Oct;23(10):701-4. doi: 10.1111/exd.12483. Epub 2014 Jul 31. Exp Dermatol. 2014. PMID: 24980461 Free PMC article. Review.
-
[Advances in apolipoprotein A- I and it's anti-atherosclerosis properties].Sheng Wu Gong Cheng Xue Bao. 2003 Jul;19(4):387-91. Sheng Wu Gong Cheng Xue Bao. 2003. PMID: 15969051 Review. Chinese.
Cited by
-
Metabolic adaptations of tissue-resident immune cells.Nat Immunol. 2019 Jul;20(7):793-801. doi: 10.1038/s41590-019-0407-0. Epub 2019 Jun 18. Nat Immunol. 2019. PMID: 31213715 Review.
-
Alternative Splicing of FN (Fibronectin) Regulates the Composition of the Arterial Wall Under Low Flow.Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):e18-e32. doi: 10.1161/ATVBAHA.120.314013. Epub 2020 Nov 19. Arterioscler Thromb Vasc Biol. 2021. PMID: 33207933 Free PMC article.
-
Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery.Int J Mol Sci. 2020 Jan 23;21(3):739. doi: 10.3390/ijms21030739. Int J Mol Sci. 2020. PMID: 31979310 Free PMC article. Review.
-
Pulmonary hypertension: Linking inflammation and pulmonary arterial stiffening.Front Immunol. 2022 Oct 5;13:959209. doi: 10.3389/fimmu.2022.959209. eCollection 2022. Front Immunol. 2022. PMID: 36275740 Free PMC article. Review.
-
A Bibliometric Analysis of Global Research Trends in Psoriasis and Metabolic Syndrome.Clin Cosmet Investig Dermatol. 2024 Feb 9;17:365-382. doi: 10.2147/CCID.S446966. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 38352064 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

