Fetuin-A protein distribution in mature inflamed and ischemic brain tissue
- PMID: 30412582
- PMCID: PMC6226147
- DOI: 10.1371/journal.pone.0206597
Fetuin-A protein distribution in mature inflamed and ischemic brain tissue
Abstract
Background: The liver-derived plasma protein fetuin-A is strongly expressed during fetal life, hence its name. Fetuin-A protein is normally present in most fetal organs and tissues, including brain tissue. Fetuin-A was neuroprotective in animal models of cerebral ischemia and lethal chronic inflammation, suggesting a role beyond the neonatal period. Little is known, however, on the presence of fetuin-A in mature human brain tissue under different physiological and pathological conditions.
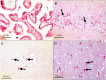
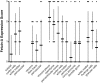
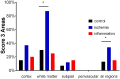
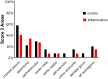
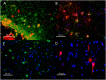
Methods: We studied by immunohistochemistry (IHC) the distribution of fetuin-A protein in mature human brain autopsy tissues from patients without neurological disease, patients with inflammatory brain disorders, and patients with ischemic brain lesions. To identify fetuin-A-positive cells in these tissues we co-localized fetuin-A with GFAP (astrocytes) and CD68 (macrophages, activated microglia).
Results and discussion: Unlike previous reports, we detected fetuin-A protein also in mature human brain as would be expected from an abundant plasma protein also present in cerebrospinal fluid. Fetuin-A immunoreactivity was increased in ischemic white matter and decreased in inflamed cerebellar tissue. Fetuin-A immunostaining was predominantly associated with neurons and astrocytes. Unlike the developing brain, the adult brain lacked fetuin-A immunostaining in CD68-positive microglia. Our findings suggest a role for fetuin-A in tissue remodeling of neonatal brain, which becomes obsolete in the adult brain, but is re-activated in damaged brain tissue. To further assess the role of fetuin-A in the mature brain, animal models involving ischemia and inflammation need to be studied.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats.Exp Neurol. 2001 Jul;170(1):63-71. doi: 10.1006/exnr.2001.7701. Exp Neurol. 2001. PMID: 11421584
-
Fetuin-a in the developing brain.Dev Neurobiol. 2013 May;73(5):354-69. doi: 10.1002/dneu.22064. Epub 2012 Nov 29. Dev Neurobiol. 2013. PMID: 23109215
-
Fluoro-Jade B staining as useful tool to identify activated microglia and astrocytes in a mouse transgenic model of Alzheimer's disease.Brain Res. 2007 Jan 12;1128(1):40-9. doi: 10.1016/j.brainres.2006.05.050. Epub 2006 Nov 27. Brain Res. 2007. PMID: 17125750
-
Anti-inflammatory role of fetuin-A in injury and infection.Curr Mol Med. 2012 Jun;12(5):625-33. doi: 10.2174/156652412800620039. Curr Mol Med. 2012. PMID: 22292896 Free PMC article. Review.
-
Glial reactions in Parkinson's disease.Mov Disord. 2008 Mar 15;23(4):474-83. doi: 10.1002/mds.21751. Mov Disord. 2008. PMID: 18044695 Review.
Cited by
-
Association Between Human Blood Metabolome and the Risk of Psychiatric Disorders.Schizophr Bull. 2023 Mar 15;49(2):428-443. doi: 10.1093/schbul/sbac130. Schizophr Bull. 2023. PMID: 36124769 Free PMC article.
-
Post-translational modifications glycosylation and phosphorylation of the major hepatic plasma protein fetuin-A are associated with CNS inflammation in children.PLoS One. 2022 Oct 7;17(10):e0268592. doi: 10.1371/journal.pone.0268592. eCollection 2022. PLoS One. 2022. PMID: 36206263 Free PMC article.
-
Fetuin-A is a HIF target that safeguards tissue integrity during hypoxic stress.Nat Commun. 2021 Jan 22;12(1):549. doi: 10.1038/s41467-020-20832-7. Nat Commun. 2021. PMID: 33483479 Free PMC article.
-
Tissue chaperoning-the expanded functions of fetuin-A beyond inhibition of systemic calcification.Pflugers Arch. 2022 Aug;474(8):949-962. doi: 10.1007/s00424-022-02688-6. Epub 2022 Apr 11. Pflugers Arch. 2022. PMID: 35403906 Free PMC article. Review.
-
The possible link between Fetuin-A Protein and Neuro-inflammation in Children with Autism Spectrum Disorder.Pak J Med Sci. 2021 Jul-Aug;37(4):1166-1171. doi: 10.12669/pjms.37.4.4032. Pak J Med Sci. 2021. PMID: 34290802 Free PMC article.
References
-
- Pedersen KO. Fetuin, a new globin isolated from serum. Nature 1944;154:570–575.
-
- Heremans JF, Lambin P. Les globulines sériques du système gamma: leur nature et leur pathologie: Editions Arscia; 1960.
-
- Burgi W, Schmid K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem. 1961;236:1066–74. . - PubMed
-
- Schultze H, Heide K, Haupt H. Charakterisierung eines niedermolekularen α 2-Mukoids aus Humanserum. Naturwissenschaften. 1962;49(1):15-.
-
- Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979;64(4):1118–29. Epub 1979/10/01. 10.1172/JCI109551 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

