The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer
- PMID: 30405882
- PMCID: PMC6201850
- DOI: 10.18632/oncotarget.26175
The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer
Abstract
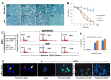
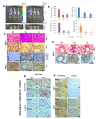
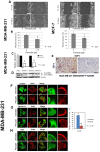
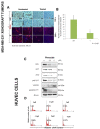
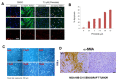
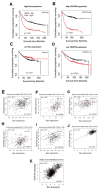
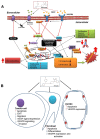
Pimozide, an antipsychotic drug of the diphenylbutylpiperidine class, has been shown to suppress cell growth of breast cancer cells in vitro. In this study we further explore the inhibitory effects of this molecule in cancer cells. We found that Pimozide inhibited cell proliferation in a dose- and time-dependent manner in MDA-MB-231 breast cancer cells and A549 lung cancer cells. Furthermore, we found that Pimozide also promoted apoptosis as demonstrated by cell cycle arrest and induction of double-strand DNA breaks but did not result in any effect in the non-transformed MCF10A breast cell line. In order to shed new lights into the molecular pathways affected by Pimozide, we show that Pimozide downregulated RAN GTPase and AKT at both protein and mRNA levels and inhibited the AKT signaling pathway in MDA-MB-231 breast cancer cells. Pimozide also inhibited the epithelial mesenchymal transition and cell migration and downregulated the expression of MMPs. Administration of Pimozide showed a potent in vivo antitumor activity in MDA-MB-231 xenograft animal model and reduced the number of lung metastases by blocking vascular endothelial growth factor receptor 2. Furthermore, Pimozide inhibited myofibroblast formation as evaluated by the reduction in α-smooth muscle actin containing cells. Thus, Pimozide might inhibit tumor development by suppressing angiogenesis and by paracrine stimulation provided by host reactive stromal cells. These results demonstrate a novel in vitro and in vivo antitumor activity of Pimozide against breast and lung cancer cells and provide the proof of concept for a putative Pimozide as a novel approach for cancer therapy.
Keywords: DSB; apoptosis; breast cancer; pimozide; xenograft.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


Similar articles
-
The antipsychotic drug pimozide inhibits cell growth in prostate cancer through suppression of STAT3 activation.Int J Oncol. 2016 Jan;48(1):322-8. doi: 10.3892/ijo.2015.3229. Epub 2015 Nov 4. Int J Oncol. 2016. PMID: 26549437
-
Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells.Toxicol Appl Pharmacol. 2008 Jan 15;226(2):178-91. doi: 10.1016/j.taap.2007.09.013. Epub 2007 Sep 21. Toxicol Appl Pharmacol. 2008. PMID: 17961621
-
The neuroleptic drug pimozide inhibits stem-like cell maintenance and tumorigenicity in hepatocellular carcinoma.Oncotarget. 2017 Mar 14;8(11):17593-17609. doi: 10.18632/oncotarget.4307. Oncotarget. 2017. PMID: 26061710 Free PMC article.
-
Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway.Drug Des Devel Ther. 2016 Apr 18;10:1419-41. doi: 10.2147/DDDT.S102541. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27143851 Free PMC article.
-
Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy.Semin Cancer Biol. 2021 Jan;68:75-83. doi: 10.1016/j.semcancer.2019.10.007. Epub 2019 Oct 13. Semin Cancer Biol. 2021. PMID: 31618686 Free PMC article. Review.
Cited by
-
In silico Selection and Experimental Validation of FDA-Approved Drugs as Anti-quorum Sensing Agents.Front Microbiol. 2019 Oct 10;10:2355. doi: 10.3389/fmicb.2019.02355. eCollection 2019. Front Microbiol. 2019. PMID: 31649658 Free PMC article.
-
Repurposing Antipsychotics for Cancer Treatment.Biomedicines. 2021 Nov 28;9(12):1785. doi: 10.3390/biomedicines9121785. Biomedicines. 2021. PMID: 34944601 Free PMC article. Review.
-
STAT5a Confers Doxorubicin Resistance to Breast Cancer by Regulating ABCB1.Front Oncol. 2021 Jul 15;11:697950. doi: 10.3389/fonc.2021.697950. eCollection 2021. Front Oncol. 2021. PMID: 34336684 Free PMC article.
-
Primary assessment of medicines for expected migrastatic potential with holographic incoherent quantitative phase imaging.Biomed Opt Express. 2023 May 16;14(6):2689-2708. doi: 10.1364/BOE.488630. eCollection 2023 Jun 1. Biomed Opt Express. 2023. PMID: 37342686 Free PMC article.
-
Pimozide-loaded nanostructured lipid carriers: Repurposing strategy against lung cancer.Sci Prog. 2024 Oct-Dec;107(4):368504241296304. doi: 10.1177/00368504241296304. Sci Prog. 2024. PMID: 39497512 Free PMC article.
References
-
- Tecott LH, Kwong LL, Uhr S, Peroutka SJ. Differential modulation of dopamine D2 receptors by chronic haloperidol, nitrendipine, and pimozide. Biol Psychiatry. 1986;21:1114–22. - PubMed
-
- Jandaghi P, Najafabadi HS, Bauer AS, Papadakis AI, Fassan M, Hall A, Monast A, von Knebel Doeberitz M, Neoptolemos JP, Costello E, Greenhalf W, Scarpa A, Sipos B, et al. Expression of DRD2 is increased in human pancreatic ductal adenocarcinoma and inhibitors slow tumor growth in mice. Gastroenterology. 2016;151:1218–31. doi: 10.1053/j.gastro.2016.08.040. - DOI - PubMed
-
- Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

