Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals
- PMID: 30404917
- PMCID: PMC6275525
- DOI: 10.1073/pnas.1719169115
Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals
Abstract
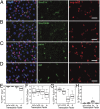
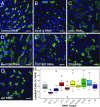
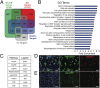
Epithelial homeostasis requires the precise balance of epithelial stem/progenitor proliferation and differentiation. While many signaling pathways that regulate epithelial stem cells have been identified, it is probable that other regulators remain unidentified. Here, we use gene-expression profiling by targeted DamID to identify the stem/progenitor-specific transcription and signaling factors in the Drosophila midgut. Many signaling pathway components, including ligands of most major pathways, exhibit stem/progenitor-specific expression and have regulatory regions bound by both intrinsic and extrinsic transcription factors. In addition to previously identified stem/progenitor-derived ligands, we show that both the insulin-like factor Ilp6 and TNF ligand eiger are specifically expressed in the stem/progenitors and regulate normal tissue homeostasis. We propose that intestinal stem cells not only integrate multiple signals but also contribute to and regulate the homeostatic signaling microenvironmental niche through the expression of autocrine and paracrine factors.
Keywords: epithelial homeostasis; insulin; microenvironment; niche; stem cells.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Nutritional regulation of stem and progenitor cells in Drosophila.Development. 2013 Dec;140(23):4647-56. doi: 10.1242/dev.079087. Development. 2013. PMID: 24255094 Free PMC article. Review.
-
ClC-c regulates the proliferation of intestinal stem cells via the EGFR signalling pathway in Drosophila.Cell Prolif. 2022 Jan;55(1):e13173. doi: 10.1111/cpr.13173. Epub 2021 Dec 24. Cell Prolif. 2022. PMID: 34952996 Free PMC article.
-
A transient niche regulates the specification of Drosophila intestinal stem cells.Science. 2010 Jan 8;327(5962):210-3. doi: 10.1126/science.1181958. Science. 2010. PMID: 20056890 Free PMC article.
-
Intestinal stem cell response to injury: lessons from Drosophila.Cell Mol Life Sci. 2016 Sep;73(17):3337-49. doi: 10.1007/s00018-016-2235-9. Epub 2016 May 2. Cell Mol Life Sci. 2016. PMID: 27137186 Free PMC article. Review.
-
Integrin signaling is required for maintenance and proliferation of intestinal stem cells in Drosophila.Dev Biol. 2013 May 1;377(1):177-87. doi: 10.1016/j.ydbio.2013.01.032. Epub 2013 Feb 8. Dev Biol. 2013. PMID: 23410794
Cited by
-
The Septate Junction Protein Tsp2A Restricts Intestinal Stem Cell Activity via Endocytic Regulation of aPKC and Hippo Signaling.Cell Rep. 2019 Jan 15;26(3):670-688.e6. doi: 10.1016/j.celrep.2018.12.079. Cell Rep. 2019. PMID: 30650359 Free PMC article.
-
Epithelial Cell Polarity During Drosophila Midgut Development.Front Cell Dev Biol. 2022 Jun 30;10:886773. doi: 10.3389/fcell.2022.886773. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35846367 Free PMC article. Review.
-
The Snakeskin-Mesh Complex of Smooth Septate Junction Restricts Yorkie to Regulate Intestinal Homeostasis in Drosophila.Stem Cell Reports. 2020 May 12;14(5):828-844. doi: 10.1016/j.stemcr.2020.03.021. Epub 2020 Apr 23. Stem Cell Reports. 2020. PMID: 32330445 Free PMC article.
-
zfh2 controls progenitor cell activation and differentiation in the adult Drosophila intestinal absorptive lineage.PLoS Genet. 2019 Dec 16;15(12):e1008553. doi: 10.1371/journal.pgen.1008553. eCollection 2019 Dec. PLoS Genet. 2019. PMID: 31841513 Free PMC article.
-
Aging-related upregulation of the homeobox gene caudal represses intestinal stem cell differentiation in Drosophila.PLoS Genet. 2021 Jul 6;17(7):e1009649. doi: 10.1371/journal.pgen.1009649. eCollection 2021 Jul. PLoS Genet. 2021. PMID: 34228720 Free PMC article.
References
-
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. - PubMed
-
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. - PubMed
-
- Doupé DP, Jones PH. Cycling progenitors maintain epithelia while diverse cell types contribute to repair. BioEssays. 2013;35:443–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

