Znf179 E3 ligase-mediated TDP-43 polyubiquitination is involved in TDP-43- ubiquitinated inclusions (UBI) (+)-related neurodegenerative pathology
- PMID: 30404641
- PMCID: PMC6223059
- DOI: 10.1186/s12929-018-0479-4
Znf179 E3 ligase-mediated TDP-43 polyubiquitination is involved in TDP-43- ubiquitinated inclusions (UBI) (+)-related neurodegenerative pathology
Abstract
Background: The brain predominantly expressed RING finger protein, Znf179, is known to be important for embryonic neuronal differentiation during brain development. Downregulation of Znf179 has been observed in motor neurons of adult mouse models for amyotrophic lateral sclerosis (ALS), yet the molecular function of Znf179 in neurodegeneration has never been previously described. Znf179 contains the classical C3HC4 RING finger domain, and numerous proteins containing C3HC4 RING finger domain act as E3 ubiquitin ligases. Hence, we are interested to identify whether Znf179 possesses E3 ligase activity and its role in ALS neuropathy.
Methods: We used in vivo and in vitro ubiquitination assay to examine the E3 ligase autoubiquitination activity of Znf179 and its effect on 26S proteasome activity. To search for the candidate substrates of Znf179, we immunoprecipitated Znf179 and subjected to mass spectrometry (MS) analysis to identify its interacting proteins. We found that ALS/ FTLD-U (frontotemporal lobar degeneration (FTLD) with ubiquitin inclusions)-related neurodegenerative TDP-43 protein is the E3 ligase substrate of Znf179. To further clarify the role of E3 ubiquitin ligase Znf179 in neurodegenerative TDP-43-UBI (ubiquitinated inclusions) (+) proteinopathy, the effect of Znf179-mediated TDP-43 polyubiquitination on TDP-43 protein stability, aggregate formation and nucleus/cytoplasm mislocalization were evaluated in vitro cell culture system and in vivo animal model.
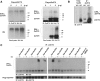
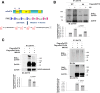
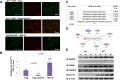
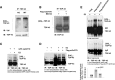
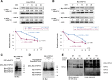
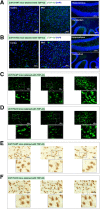
Results: Here we report that Znf179 is a RING E3 ubiquitin ligase which possesses autoubiquitination feature and regulates 26S proteasome activity through modulating the protein expression levels of 19S/20S proteasome subunits. Our immunoprecipitation assay and MS analysis results revealed that the neuropathological TDP-43 protein is one of its E3 ligase substrate. Znf179 interactes with TDP-43 protein and mediates polyubiquitination of TDP-43 in vitro and in vivo. In neurodegenerative TDP-43 proteinopathy, we found that Znf179-mediated polyubiquitination of TDP-43 accelerates its protein turnover rate and attenuates insoluble pathologic TDP-43 aggregates, while knockout of Znf179 in mouse brain results in accumulation of insoluble TDP-43 and cytosolic TDP-43 inclusions in cortex, hippocampus and midbrain regions.
Conclusions: Here we unveil the important role for the novel E3 ligase Znf179 in TDP-43-mediated neuropathy, and provide a potential therapeutic strategy for combating ALS/ FTLD-U neurodegenerative pathologies.
Keywords: ALS; E3 ligase; FTLD-U; Polyubiquitination; TDP-43; Znf179.
Conflict of interest statement
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Praja1 RING-finger E3 ubiquitin ligase suppresses neuronal cytoplasmic TDP-43 aggregate formation.Neuropathology. 2020 Dec;40(6):570-586. doi: 10.1111/neup.12694. Epub 2020 Jul 19. Neuropathology. 2020. PMID: 32686212 Free PMC article.
-
Therapeutic effect of berberine on TDP-43-related pathogenesis in FTLD and ALS.J Biomed Sci. 2016 Oct 21;23(1):72. doi: 10.1186/s12929-016-0290-z. J Biomed Sci. 2016. PMID: 27769241 Free PMC article.
-
Regionally different immunoreactivity for Smurf2 and pSmad2/3 in TDP-43-positive inclusions of amyotrophic lateral sclerosis.Neuropathol Appl Neurobiol. 2013 Feb;39(2):144-56. doi: 10.1111/j.1365-2990.2012.01270.x. Neuropathol Appl Neurobiol. 2013. PMID: 22435645
-
[Neurodegenerative disorders and TDP-43].Brain Nerve. 2009 Feb;61(2):161-6. Brain Nerve. 2009. PMID: 19235466 Review. Japanese.
-
ALS and FTLD: two faces of TDP-43 proteinopathy.Eur J Neurol. 2008 Aug;15(8):772-80. doi: 10.1111/j.1468-1331.2008.02195.x. Eur J Neurol. 2008. PMID: 18684309 Free PMC article. Review.
Cited by
-
Post-Translational Variants of Major Proteins in Amyotrophic Lateral Sclerosis Provide New Insights into the Pathophysiology of the Disease.Int J Mol Sci. 2024 Aug 8;25(16):8664. doi: 10.3390/ijms25168664. Int J Mol Sci. 2024. PMID: 39201350 Free PMC article. Review.
-
Functional implication of ubiquitinating and deubiquitinating mechanisms in TDP-43 proteinopathies.Front Cell Dev Biol. 2022 Sep 9;10:931968. doi: 10.3389/fcell.2022.931968. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158183 Free PMC article. Review.
-
Specificity Protein 1: A Protein With a Two-Sided Role in Ischemic Stroke.Front Cell Neurosci. 2021 Dec 14;15:757670. doi: 10.3389/fncel.2021.757670. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34970121 Free PMC article. Review.
-
Is PROTAC technology really a game changer for central nervous system drug discovery?Expert Opin Drug Discov. 2021 Aug;16(8):833-840. doi: 10.1080/17460441.2021.1915979. Epub 2021 Apr 19. Expert Opin Drug Discov. 2021. PMID: 33870803 Free PMC article.
-
Multiple distinct pathways lead to hyperubiquitylated insoluble TDP-43 protein independent of its translocation into stress granules.J Biol Chem. 2020 Jan 17;295(3):673-689. doi: 10.1074/jbc.RA119.010617. Epub 2019 Nov 28. J Biol Chem. 2020. PMID: 31780563 Free PMC article.
References
-
- Mackenzie IR, Feldman HH. Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol. 2005;64:730–739. doi: 10.1097/01.jnen.0000174335.27708.0a. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

