Effects of Common CYP1A2 Genotypes and Other Key Factors on Intraindividual Variation in the Caffeine Metabolic Ratio: An Exploratory Analysis
- PMID: 30387917
- PMCID: PMC6342244
- DOI: 10.1111/cts.12598
Effects of Common CYP1A2 Genotypes and Other Key Factors on Intraindividual Variation in the Caffeine Metabolic Ratio: An Exploratory Analysis
Abstract
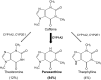
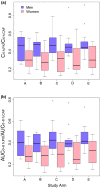
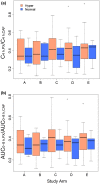
The caffeine metabolic ratio is an established marker for cytochrome P450 (CYP) 1A2 activity. Optimal sample size calculation for clinical pharmacokinetic xenobiotic-caffeine interaction studies requires robust estimates of interindividual and intraindividual variation in this ratio. Compared with interindividual variation, factors contributing to intraindividual variation are less defined. An exploratory analysis involving healthy nonsmoking non-naïve caffeine drinkers (1-3 cups/day; 12 men, 12 women) administered caffeine (160 mg) on five occasions evaluated the effects of CYP1A2 induction status (based on genotype) and other factors on intraindividual variation in CYP1A2 activity. Results were compared with those from previous studies. Regardless of whether a hyperinducer (CYP1A2*1A/*1F or CYP1A2*1F/*1F) or normal metabolizer (CYP1A2*1A/*1A, CYP1A2*1C/*1F, or CYP1A2*1C*1F/*1C*1F), sex, age, oral contraceptive use by women, and smoking status, intraindividual variation was ≤30%. A value of 30% is proposed for optimal design of pharmacokinetic xenobiotic-caffeine interaction studies. Prospective studies are needed for confirmation.
© 2018 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Figures
Similar articles
-
Quercetin significantly inhibits the metabolism of caffeine, a substrate of cytochrome P450 1A2 unrelated to CYP1A2*1C (-2964G>A) and *1F (734C>A) gene polymorphisms.Biomed Res Int. 2014;2014:405071. doi: 10.1155/2014/405071. Epub 2014 Jun 15. Biomed Res Int. 2014. PMID: 25025048 Free PMC article.
-
Inducibility of CYP1A2 by omeprazole in vivo related to the genetic polymorphism of CYP1A2.Br J Clin Pharmacol. 2002 Nov;54(5):540-3. doi: 10.1046/j.1365-2125.2002.01686.x. Br J Clin Pharmacol. 2002. PMID: 12445035 Free PMC article. Clinical Trial.
-
Effect of caffeine intake 12 or 24 hours prior to melatonin intake and CYP1A2*1F polymorphism on CYP1A2 phenotyping by melatonin.Basic Clin Pharmacol Toxicol. 2006 Oct;99(4):300-4. doi: 10.1111/j.1742-7843.2006.pto_491.x. Basic Clin Pharmacol Toxicol. 2006. PMID: 17040215
-
Assessment of CYP1A2 activity in clinical practice: why, how, and when?Basic Clin Pharmacol Toxicol. 2005 Sep;97(3):125-34. doi: 10.1111/j.1742-7843.2005.pto_973160.x. Basic Clin Pharmacol Toxicol. 2005. PMID: 16128905 Review.
-
Human cytochrome P4501A2.IARC Sci Publ. 1999;(148):173-95. IARC Sci Publ. 1999. PMID: 10493258 Review.
Cited by
-
The pharmacogenetics of the new-generation antipsychotics - A scoping review focused on patients with severe psychiatric disorders.Front Psychiatry. 2023 Feb 16;14:1124796. doi: 10.3389/fpsyt.2023.1124796. eCollection 2023. Front Psychiatry. 2023. PMID: 36873203 Free PMC article.
-
CYP1A2 expression rather than genotype is associated with olanzapine concentration in psychiatric patients.Sci Rep. 2023 Oct 28;13(1):18507. doi: 10.1038/s41598-023-45752-6. Sci Rep. 2023. PMID: 37898643 Free PMC article.
-
CYP1A2 mRNA Expression Rather than Genetic Variants Indicate Hepatic CYP1A2 Activity.Pharmaceutics. 2022 Feb 27;14(3):532. doi: 10.3390/pharmaceutics14030532. Pharmaceutics. 2022. PMID: 35335907 Free PMC article.
-
Semi-physiological Pharmacokinetic Model of Clozapine and Norclozapine in Healthy, Non-smoking Volunteers: The Impact of Race and Genetics.CNS Drugs. 2024 Jul;38(7):571-581. doi: 10.1007/s40263-024-01092-1. Epub 2024 Jun 5. CNS Drugs. 2024. PMID: 38836990
-
Determination of Gastric Water Emptying in Fasted and Fed State Conditions Using a Compression-Coated Tablet and Salivary Caffeine Kinetics.Pharmaceutics. 2023 Nov 4;15(11):2584. doi: 10.3390/pharmaceutics15112584. Pharmaceutics. 2023. PMID: 38004563 Free PMC article.
References
-
- Zanger, U.M. , Turpeinen, M. , Klein, K. & Schwab, M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 6, 1093–1108 (2008). - PubMed
-
- Kalow, W. & Tang, B.K. The use of caffeine for enzyme assays: a critical appraisal. Clin. Pharmacol. Ther. 5, 503–514 (1993). - PubMed
-
- Fredholm, B.B. , Battig, K. , Holmen, J. , Nehlig, A. & Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1, 83–133 (1999). - PubMed
-
- Food and Drug Administration Center for Drug Evaluation and Research . Clinical Drug Interaction Studies—Study Design, Data Analysis, and Clinical Implications Guidance for Industry (Draft Guidance) (US Food and Drug Administration, Silver Spring, MD, 2017).
-
- Perera, V. , Gross, A.S. & McLachlan, A.J. Measurement of CYP1A2 activity: a focus on caffeine as a probe. Curr. Drug Metab. 5, 667–678 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

