IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment
- PMID: 30377293
- PMCID: PMC6207563
- DOI: 10.1038/s41419-018-1143-3
IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment
Abstract
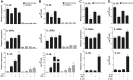
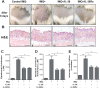
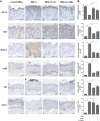
IL-36 cytokines, a subgroup of IL-1 family, comprise IL-36α, IL-36β, and IL-36γ agonists, abundantly expressed in psoriatic skin, and IL-36RA and IL-38 antagonists. In psoriatic skin, IL-36 cytokines interfere with keratinocyte cornification programs and induce the release of antimicrobial peptides and chemokines active on neutrophils and Th17 lymphocytes. To date, the role of IL-38 antagonist in psoriasis remains to be defined. Here, we demonstrate that skin and circulating IL-38 levels are reduced in psoriatic patients and in other skin diseases characterized by neutrophilic infiltrate. In psoriasis, the balance of IL-36γ agonist/IL-38 antagonist serum levels is in favor of agonists and is closely associated with disease severity. Interestingly, IL-38 is upregulated by anti-IL-17A biological treatment and positively correlates with the therapeutic efficacy of secukinumab in psoriatic patients. The downregulation of IL-38 expression is strictly related to keratinocyte de-differentiation triggered by the inflammatory cytokines IL-36γ, IL-17, and IL-22. Finally, we demonstrate that administration of recombinant full-length IL-38 counteracts in vitro the biological processes induced by IL-36γ in human keratinocytes and endothelial cells and attenuates in vivo the severity of the psoriasiform phenotype induced by IMQ in mice. Such effects are achieved by restoring the physiological programs of keratinocyte proliferation and differentiation, and reducing the immune cell infiltrates.
Conflict of interest statement
The authors declare no conflict of interests.
Figures


Similar articles
-
IL-36γ sustains a proinflammatory self-amplifying loop with IL-17C in anti-TNF-induced psoriasiform skin lesions of patients with Crohn's disease.Inflamm Bowel Dis. 2014 Nov;20(11):1891-901. doi: 10.1097/MIB.0000000000000198. Inflamm Bowel Dis. 2014. PMID: 25299544
-
Distinct expression of interleukin (IL)-36α, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn's disease.Clin Exp Immunol. 2016 May;184(2):159-73. doi: 10.1111/cei.12761. Epub 2016 Feb 22. Clin Exp Immunol. 2016. PMID: 26701127 Free PMC article.
-
Acitretin inhibits IL-17A-induced IL-36 expression in keratinocytes by down-regulating IκBζ.Int Immunopharmacol. 2020 Feb;79:106045. doi: 10.1016/j.intimp.2019.106045. Epub 2019 Dec 25. Int Immunopharmacol. 2020. PMID: 31863918
-
The Significance of IL-36 Hyperactivation and IL-36R Targeting in Psoriasis.Int J Mol Sci. 2019 Jul 5;20(13):3318. doi: 10.3390/ijms20133318. Int J Mol Sci. 2019. PMID: 31284527 Free PMC article. Review.
-
The role of IL-17 in psoriasis.J Dermatolog Treat. 2015 Feb;26(1):41-4. doi: 10.3109/09546634.2013.879093. Epub 2014 Feb 20. J Dermatolog Treat. 2015. PMID: 24552504 Review.
Cited by
-
IL-38 Gene Deletion Worsens Murine Colitis.Front Immunol. 2022 May 26;13:840719. doi: 10.3389/fimmu.2022.840719. eCollection 2022. Front Immunol. 2022. PMID: 35693797 Free PMC article.
-
Interleukin role in the regulation of endothelial cell pathological activation.Vasc Biol. 2021 Oct 18;3(1):R96-R105. doi: 10.1530/VB-21-0010. eCollection 2021. Vasc Biol. 2021. PMID: 34870094 Free PMC article. Review.
-
IL-38: A novel cytokine in systemic lupus erythematosus pathogenesis.J Cell Mol Med. 2020 Nov;24(21):12379-12389. doi: 10.1111/jcmm.15737. Epub 2020 Oct 20. J Cell Mol Med. 2020. PMID: 33079487 Free PMC article.
-
Immunobiological Properties and Clinical Applications of Interleukin-38 for Immune-Mediated Disorders: A Systematic Review Study.Int J Mol Sci. 2021 Nov 21;22(22):12552. doi: 10.3390/ijms222212552. Int J Mol Sci. 2021. PMID: 34830435 Free PMC article. Review.
-
Interleukin-36 in Infectious and Inflammatory Skin Diseases.Front Immunol. 2019 May 24;10:1162. doi: 10.3389/fimmu.2019.01162. eCollection 2019. Front Immunol. 2019. PMID: 31191535 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

