Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways
- PMID: 30352166
- PMCID: PMC6423099
- DOI: 10.1164/rccm.201804-0734OC
Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways
Abstract
Rationale: MUC5AC and MUC5B are the predominant gel-forming mucins in the mucus layer of human airways. Each mucin has distinct functions and site-specific expression. However, the regional distribution of expression and cell types that secrete each mucin in normal/healthy human airways are not fully understood.
Objectives: To characterize the regional distribution of MUC5B and MUC5AC in normal/healthy human airways and assess which cell types produce these mucins, referenced to the club cell secretory protein (CCSP).
Methods: Multiple airway regions from 16 nonsmoker lungs without a history of lung disease were studied. MUC5AC, MUC5B, and CCSP expression/colocalization were assessed by RNA in situ hybridization and immunohistochemistry in five lungs with histologically healthy airways. Droplet digital PCR and cell cultures were performed for absolute quantification of MUC5AC/5B ratios and protein secretion, respectively.
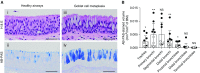
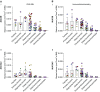
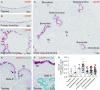
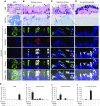
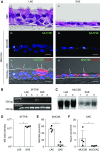
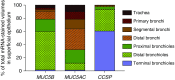
Measurements and main results: Submucosal glands expressed MUC5B, but not MUC5AC. However, MUC5B was also extensively expressed in superficial epithelia throughout the airways except for the terminal bronchioles. Morphometric calculations revealed that the distal airway superficial epithelium was the predominant site for MUC5B expression, whereas MUC5AC expression was concentrated in proximal, cartilaginous airways. RNA in situ hybridization revealed MUC5AC and MUC5B were colocalized with CCSP-positive secretory cells in proximal superficial epithelia, whereas MUC5B and CCSP-copositive cells dominated distal regions.
Conclusions: In normal/healthy human airways, MUC5B is the dominant secretory mucin in the superficial epithelium and glands, with distal airways being a major site of expression. MUC5B and MUC5AC expression is a property of CCSP-positive secretory cells in superficial airway epithelia.
Keywords: MUC5AC; MUC5B; airway mucins; club cells; distal airways.
Figures


Comment in
-
Putting Mucins on the Map.Am J Respir Crit Care Med. 2019 Mar 15;199(6):681-682. doi: 10.1164/rccm.201809-1818ED. Am J Respir Crit Care Med. 2019. PMID: 30376352 Free PMC article. No abstract available.
Similar articles
-
Mucins MUC5AC and MUC5B Are Variably Packaged in the Same and in Separate Secretory Granules.Am J Respir Crit Care Med. 2022 Nov 1;206(9):1081-1095. doi: 10.1164/rccm.202202-0309OC. Am J Respir Crit Care Med. 2022. PMID: 35776514 Free PMC article.
-
Gel-forming mucins form distinct morphologic structures in airways.Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):6842-6847. doi: 10.1073/pnas.1703228114. Epub 2017 Jun 12. Proc Natl Acad Sci U S A. 2017. PMID: 28607090 Free PMC article.
-
Muc5b is mainly expressed and sialylated in the nasal olfactory epithelium whereas Muc5ac is exclusively expressed and fucosylated in the nasal respiratory epithelium.Histochem Cell Biol. 2019 Aug;152(2):167-174. doi: 10.1007/s00418-019-01785-5. Epub 2019 Apr 27. Histochem Cell Biol. 2019. PMID: 31030254
-
Mucus hypersecretion in asthma: causes and effects.Curr Opin Pulm Med. 2009 Jan;15(1):4-11. doi: 10.1097/MCP.0b013e32831da8d3. Curr Opin Pulm Med. 2009. PMID: 19077699 Free PMC article. Review.
-
Defensive Properties of Mucin Glycoproteins during Respiratory Infections-Relevance for SARS-CoV-2.mBio. 2020 Nov 12;11(6):e02374-20. doi: 10.1128/mBio.02374-20. mBio. 2020. PMID: 33184103 Free PMC article. Review.
Cited by
-
The airway epithelium during infancy and childhood: A complex multicellular immune barrier. Basic review for clinicians.Paediatr Respir Rev. 2021 Jun;38:9-15. doi: 10.1016/j.prrv.2021.04.002. Epub 2021 Apr 22. Paediatr Respir Rev. 2021. PMID: 34030977 Free PMC article. Review.
-
The Mucin Gene MUC5B Is Required for Normal Lung Function.Am J Respir Crit Care Med. 2022 Apr 1;205(7):737-739. doi: 10.1164/rccm.202201-0064ED. Am J Respir Crit Care Med. 2022. PMID: 35148488 Free PMC article. No abstract available.
-
Network Pharmacological Analysis on the Herbal Combinations for Mitigating Inflammation in Respiratory Tracts and Experimental Evaluation.Healthcare (Basel). 2023 Jan 3;11(1):143. doi: 10.3390/healthcare11010143. Healthcare (Basel). 2023. PMID: 36611603 Free PMC article.
-
SARS-CoV-2 Induces Expression of Cytokine and MUC5AC/5B in Human Nasal Epithelial Cell through ACE 2 Receptor.Biomed Res Int. 2022 Jun 2;2022:2743046. doi: 10.1155/2022/2743046. eCollection 2022. Biomed Res Int. 2022. PMID: 35692597 Free PMC article.
-
Lung Regeneration: Cells, Models, and Mechanisms.Cold Spring Harb Perspect Biol. 2022 Oct 3;14(10):a040873. doi: 10.1101/cshperspect.a040873. Cold Spring Harb Perspect Biol. 2022. PMID: 34750172 Free PMC article. Review.
References
-
- Buisine MP, Devisme L, Copin MC, Durand-Réville M, Gosselin B, Aubert JP, et al. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol. 1999;20:209–218. - PubMed
-
- Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41:1479–1485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

