Role of PDGF receptor-α during human cytomegalovirus entry into fibroblasts
- PMID: 30275317
- PMCID: PMC6196492
- DOI: 10.1073/pnas.1806305115
Role of PDGF receptor-α during human cytomegalovirus entry into fibroblasts
Abstract
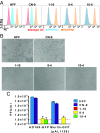
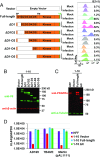
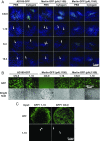
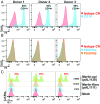
Human CMV (HCMV) exhibits a broad cell tropism that depends on two virion glycoprotein complexes: a trimeric complex (gH/gL/gO) that facilitates viral infection primarily in fibroblasts and a pentameric complex (gH/gL/pUL128-pUL130-pUL131A) that mediates infection in epithelial and endothelial cells. We performed genome-wide CRISPR screens in which the PDGF receptor-α (PDGFRα) was identified as the most significant cellular gene product essential for infection by HCMV virions containing only trimeric complex (trimer-only virus). Trimer-only virus did not enter PDGFRα knockout fibroblasts. By using knockout fibroblasts, the extracellular domain of PDGFRα required for virus entry was mapped, and the intracellular tyrosine kinase domain was shown to be nonessential. In addition, direct cell-to-cell spread of virus from knockout cells transfected with trimer-only viral DNA was blocked, despite the production of infectious virus in the transfected cells. In contrast to trimer-only virus, wild-type HCMV virions containing both trimeric and pentameric complexes entered PDGFRα knockout cells, reinforcing the view that fibroblasts contain a second, independent receptor for the pentameric complex. Importantly, however, wild-type virus entered the knockout fibroblasts at reduced efficiency compared with parental fibroblasts, arguing that the cellular receptor for the virion pentameric complex is limiting or that virions are produced containing different relative amounts of the two glycoprotein complexes. Finally, ectopic expression of PDGFRα in ARPE-19 epithelial cells and THP-1 monocytic cells, which have little to no endogenous PDGFRα expression, markedly enhanced their susceptibility to trimer-only virions. In sum, our data clarify several key determinants of HCMV tropism.
Keywords: cellular receptor; herpesvirus; tropism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
The N Terminus of Human Cytomegalovirus Glycoprotein O Is Important for Binding to the Cellular Receptor PDGFRα.J Virol. 2019 May 15;93(11):e00138-19. doi: 10.1128/JVI.00138-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894468 Free PMC article.
-
Differential Expression of PDGF Receptor-α in Human Placental Trophoblasts Leads to Different Entry Pathways by Human Cytomegalovirus Strains.Sci Rep. 2020 Jan 23;10(1):1082. doi: 10.1038/s41598-020-57471-3. Sci Rep. 2020. PMID: 31974453 Free PMC article.
-
Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry.PLoS Pathog. 2017 Apr 12;13(4):e1006281. doi: 10.1371/journal.ppat.1006281. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28403202 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
Cited by
-
Inclusion of the Viral Pentamer Complex in a Vaccine Design Greatly Improves Protection against Congenital Cytomegalovirus in the Guinea Pig Model.J Virol. 2019 Oct 29;93(22):e01442-19. doi: 10.1128/JVI.01442-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31484753 Free PMC article.
-
Endoplasmic Reticulum (ER) Reorganization and Intracellular Retention of CD58 Are Functionally Independent Properties of the Human Cytomegalovirus ER-Resident Glycoprotein UL148.J Virol. 2020 Feb 14;94(5):e01435-19. doi: 10.1128/JVI.01435-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801856 Free PMC article.
-
Cytomegalovirus Strain TB40/E Restrictions and Adaptations to Growth in ARPE-19 Epithelial Cells.Microorganisms. 2020 Apr 24;8(4):615. doi: 10.3390/microorganisms8040615. Microorganisms. 2020. PMID: 32344555 Free PMC article.
-
Distinct early role of PTEN regulation during HCMV infection of monocytes.Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2312290121. doi: 10.1073/pnas.2312290121. Epub 2024 Mar 14. Proc Natl Acad Sci U S A. 2024. PMID: 38483999 Free PMC article.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
References
-
- Shenk TE, Stinski MF, editors. Human Cytomegalovirus. Springer; Berlin: 2008. - PubMed
-
- Sinzger C, Digel M, Jahn G. Human Cytomegalovirus, Current Topics in Microbiology and Immunology. Springer; Berlin: 2008. Cytomegalovirus cell tropism; pp. 63–83. - PubMed
-
- Britt W. Human Cytomegalovirus, Current Topics in Microbiology and Immunology. Springer; Berlin: 2008. Manifestations of human cytomegalovirus infection: Proposed mechanisms of acute and chronic disease; pp. 417–470. - PubMed
-
- Gerna G, Revello MG, Baldanti F, Percivalle E, Lilleri D. The pentameric complex of human cytomegalovirus: Cell tropism, virus dissemination, immune response and vaccine development. J Gen Virol. 2017;98:2215–2234. - PubMed
-
- Podlech J, Reddehase MJ, Adler B, Lemmermann NAW. Principles for studying in vivo attenuation of virus mutants: Defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm. Med Microbiol Immunol. 2015;204:295–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

