Talin as a mechanosensitive signaling hub
- PMID: 30254032
- PMCID: PMC6219721
- DOI: 10.1083/jcb.201808061
Talin as a mechanosensitive signaling hub
Abstract
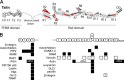
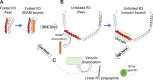
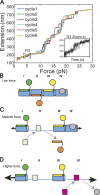
Cell adhesion to the extracellular matrix (ECM), mediated by transmembrane receptors of the integrin family, is exquisitely sensitive to biochemical, structural, and mechanical features of the ECM. Talin is a cytoplasmic protein consisting of a globular head domain and a series of α-helical bundles that form its long rod domain. Talin binds to the cytoplasmic domain of integrin β-subunits, activates integrins, couples them to the actin cytoskeleton, and regulates integrin signaling. Recent evidence suggests switch-like behavior of the helix bundles that make up the talin rod domains, where individual domains open at different tension levels, exerting positive or negative effects on different protein interactions. These results lead us to propose that talin functions as a mechanosensitive signaling hub that integrates multiple extracellular and intracellular inputs to define a major axis of adhesion signaling.
© 2018 Goult et al.
Figures

Similar articles
-
Force-Directed "Mechanointeractome" of Talin-Integrin.Biochemistry. 2019 Nov 26;58(47):4677-4695. doi: 10.1021/acs.biochem.9b00442. Epub 2019 Aug 21. Biochemistry. 2019. PMID: 31393109 Review.
-
Talin in mechanotransduction and mechanomemory at a glance.J Cell Sci. 2021 Oct 15;134(20):jcs258749. doi: 10.1242/jcs.258749. Epub 2021 Oct 28. J Cell Sci. 2021. PMID: 34708856 Free PMC article.
-
Talin‑1 interaction network in cellular mechanotransduction (Review).Int J Mol Med. 2022 May;49(5):60. doi: 10.3892/ijmm.2022.5116. Epub 2022 Mar 10. Int J Mol Med. 2022. PMID: 35266014 Free PMC article. Review.
-
The domain structure of talin: residues 1815-1973 form a five-helix bundle containing a cryptic vinculin-binding site.FEBS Lett. 2010 Jun 3;584(11):2237-41. doi: 10.1016/j.febslet.2010.04.028. Epub 2010 Apr 20. FEBS Lett. 2010. PMID: 20399778 Free PMC article.
-
Talin mechanosensitivity is modulated by a direct interaction with cyclin-dependent kinase-1.J Biol Chem. 2021 Jul;297(1):100837. doi: 10.1016/j.jbc.2021.100837. Epub 2021 Jun 9. J Biol Chem. 2021. PMID: 34118235 Free PMC article.
Cited by
-
An efficient alpha helix model and simulation framework for stationary electrostatic interaction force estimation.Sci Rep. 2021 Apr 27;11(1):9053. doi: 10.1038/s41598-021-88369-3. Sci Rep. 2021. PMID: 33907198 Free PMC article.
-
Piezo1 Channels Contribute to the Regulation of Human Atrial Fibroblast Mechanical Properties and Matrix Stiffness Sensing.Cells. 2021 Mar 16;10(3):663. doi: 10.3390/cells10030663. Cells. 2021. PMID: 33809739 Free PMC article.
-
Nano-Precision Tweezers for Mechanosensitive Proteins and Beyond.Mol Cells. 2022 Jan 31;45(1):16-25. doi: 10.14348/molcells.2022.2026. Mol Cells. 2022. PMID: 35114644 Free PMC article. Review.
-
Isolated THATCH domain of End4 is unable to bind F-actin independently in the fission yeast Schizosaccharomyces pombe.MicroPubl Biol. 2022 Jan 6;2022:10.17912/micropub.biology.000508. doi: 10.17912/micropub.biology.000508. eCollection 2022. MicroPubl Biol. 2022. PMID: 35024575 Free PMC article.
-
Differential stiffness between brain vasculature and parenchyma promotes metastatic infiltration through vessel co-option.Nat Cell Biol. 2024 Dec;26(12):2144-2153. doi: 10.1038/s41556-024-01532-6. Epub 2024 Oct 24. Nat Cell Biol. 2024. PMID: 39448802
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

