Proline provides site-specific flexibility for in vivo collagen
- PMID: 30218106
- PMCID: PMC6138679
- DOI: 10.1038/s41598-018-31937-x
Proline provides site-specific flexibility for in vivo collagen
Abstract
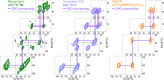
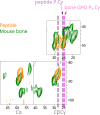
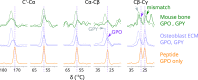
Fibrillar collagens have mechanical and biological roles, providing tissues with both tensile strength and cell binding sites which allow molecular interactions with cell-surface receptors such as integrins. A key question is: how do collagens allow tissue flexibility whilst maintaining well-defined ligand binding sites? Here we show that proline residues in collagen glycine-proline-hydroxyproline (Gly-Pro-Hyp) triplets provide local conformational flexibility, which in turn confers well-defined, low energy molecular compression-extension and bending, by employing two-dimensional 13C-13C correlation NMR spectroscopy on 13C-labelled intact ex vivo bone and in vitro osteoblast extracellular matrix. We also find that the positions of Gly-Pro-Hyp triplets are highly conserved between animal species, and are spatially clustered in the currently-accepted model of molecular ordering in collagen type I fibrils. We propose that the Gly-Pro-Hyp triplets in fibrillar collagens provide fibril "expansion joints" to maintain molecular ordering within the fibril, thereby preserving the structural integrity of ligand binding sites.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Collagen Structure-Function Relationships from Solid-State NMR Spectroscopy.Acc Chem Res. 2018 Jul 17;51(7):1621-1629. doi: 10.1021/acs.accounts.8b00092. Epub 2018 Jun 22. Acc Chem Res. 2018. PMID: 29931970 Review.
-
Hydroxyproline Ring Pucker Causes Frustration of Helix Parameters in the Collagen Triple Helix.Sci Rep. 2015 Jul 29;5:12556. doi: 10.1038/srep12556. Sci Rep. 2015. PMID: 26220399 Free PMC article.
-
Conformational effects of Gly-X-Gly interruptions in the collagen triple helix.J Mol Biol. 2006 Sep 15;362(2):298-311. doi: 10.1016/j.jmb.2006.07.014. Epub 2006 Jul 15. J Mol Biol. 2006. PMID: 16919298
-
The peptides acetyl-(Gly-3(S)Hyp-4(R)Hyp)10-NH2 and acetyl-(Gly-Pro-3(S)Hyp)10-NH2 do not form a collagen triple helix.J Biol Chem. 2004 Jan 2;279(1):282-7. doi: 10.1074/jbc.M308181200. Epub 2003 Oct 23. J Biol Chem. 2004. PMID: 14576161
-
Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth.Amino Acids. 2018 Jan;50(1):29-38. doi: 10.1007/s00726-017-2490-6. Epub 2017 Sep 20. Amino Acids. 2018. PMID: 28929384 Review.
Cited by
-
Collagen- and hyaluronic acid-based hydrogels and their biomedical applications.Mater Sci Eng R Rep. 2021 Oct;146:100641. doi: 10.1016/j.mser.2021.100641. Epub 2021 Jul 30. Mater Sci Eng R Rep. 2021. PMID: 34483486 Free PMC article.
-
Host-emitted amino acid cues regulate bacterial chemokinesis to enhance colonization.Cell Host Microbe. 2021 Aug 11;29(8):1221-1234.e8. doi: 10.1016/j.chom.2021.06.003. Epub 2021 Jul 6. Cell Host Microbe. 2021. PMID: 34233153 Free PMC article.
-
Increased Collagen Turnover Impairs Tendon Microstructure and Stability in Integrin α2β1-Deficient Mice.Int J Mol Sci. 2020 Apr 18;21(8):2835. doi: 10.3390/ijms21082835. Int J Mol Sci. 2020. PMID: 32325713 Free PMC article.
-
Consequences of incorporating thiaproline and its oxidized derivatives into collagen triple helices.Protein Sci. 2023 Jun;32(6):e4650. doi: 10.1002/pro.4650. Protein Sci. 2023. PMID: 37132632 Free PMC article.
-
Molecular conformations and dynamics in the extracellular matrix of mammalian structural tissues: Solid-state NMR spectroscopy approaches.Matrix Biol Plus. 2021 Oct 6;12:100086. doi: 10.1016/j.mbplus.2021.100086. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34746737 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

