Leutusome: A Biomimetic Nanoplatform Integrating Plasma Membrane Components of Leukocytes and Tumor Cells for Remarkably Enhanced Solid Tumor Homing
- PMID: 30207473
- PMCID: PMC6292712
- DOI: 10.1021/acs.nanolett.8b01892
Leutusome: A Biomimetic Nanoplatform Integrating Plasma Membrane Components of Leukocytes and Tumor Cells for Remarkably Enhanced Solid Tumor Homing
Abstract
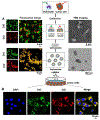
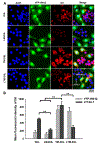
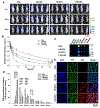
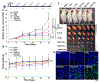
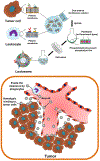
Cell membrane-camouflaged nanoparticles have appeared as a promising platform to develop active tumor targeting nanomedicines. To evade the immune surveillance, we designed a composite cell membrane-camouflaged biomimetic nanoplatform, namely, leutusome, which is made of liposomal nanoparticles incorporating plasma membrane components derived from both leukocytes (murine J774A.1 cells) and tumor cells (head and neck tumor cells HN12). Exogenous phospholipids were used as building blocks to fuse with two cell membranes to form liposomal nanoparticles. Liposomal nanoparticles made of exogenous phospholipids only or in combination with one type of cell membrane were fabricated and compared. The anticancer drug paclitaxel (PTX) was used to make drug-encapsulating liposomal nanoparticles. Leutusome resembling characteristic plasma membrane components of the two cell membranes were examined and confirmed in vitro. A xenograft mouse model of head and neck cancer was used to profile the blood clearance kinetics, biodistribution, and antitumor efficacy of the different liposomal nanoparticles. The results demonstrated that leutusome obtained prolonged blood circulation and was most efficient accumulating at the tumor site (79.1 ± 6.6% ID per gram of tumor). Similarly, leutusome composed of membrane fractions of B16 melanoma cells and leukocytes (J774A.1) showed prominent accumulation within the B16 tumor, suggesting the generalization of the approach. Furthermore, PTX-encapsulating leutusome was found to most potently inhibit tumor growth while not causing systemic adverse effects.
Keywords: Active targeting; cell membrane camouflage; leukocytes; nanoparticles; tumor cells; tumor microenvironment.
Conflict of interest statement
The authors declare no competing interest.
Figures
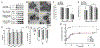
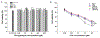

Similar articles
-
Paclitaxel-Loaded Macrophage Membrane Camouflaged Albumin Nanoparticles for Targeted Cancer Therapy.Int J Nanomedicine. 2020 Mar 19;15:1915-1928. doi: 10.2147/IJN.S244849. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32256068 Free PMC article.
-
Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy.Theranostics. 2020 Feb 10;10(7):3325-3339. doi: 10.7150/thno.41228. eCollection 2020. Theranostics. 2020. PMID: 32194871 Free PMC article.
-
Cell membrane-camouflaged liposomes for tumor cell-selective glycans engineering and imaging in vivo.Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2022769118. doi: 10.1073/pnas.2022769118. Proc Natl Acad Sci U S A. 2021. PMID: 34301864 Free PMC article.
-
Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics.Clin Transl Med. 2021 Feb;11(2):e292. doi: 10.1002/ctm2.292. Clin Transl Med. 2021. PMID: 33635002 Free PMC article. Review.
-
Biomimetic fabrication of nanotherapeutics by leukocyte membrane cloaking for targeted therapy.Colloids Surf B Biointerfaces. 2022 Nov;219:112803. doi: 10.1016/j.colsurfb.2022.112803. Epub 2022 Aug 24. Colloids Surf B Biointerfaces. 2022. PMID: 36084510 Review.
Cited by
-
Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications.Nanomicro Lett. 2019 Nov 16;11(1):100. doi: 10.1007/s40820-019-0330-9. Nanomicro Lett. 2019. PMID: 34138027 Free PMC article. Review.
-
Intensity-adjustable pain management with prolonged duration based on phase-transitional nanoparticles-assisted ultrasound imaging-guided nerve blockade.J Nanobiotechnology. 2022 Nov 24;20(1):498. doi: 10.1186/s12951-022-01707-z. J Nanobiotechnology. 2022. PMID: 36424657 Free PMC article.
-
All-stage targeted therapy for the brain metastasis from triple-negative breast cancer.Acta Pharm Sin B. 2023 Jan;13(1):359-371. doi: 10.1016/j.apsb.2022.03.026. Epub 2022 Apr 6. Acta Pharm Sin B. 2023. PMID: 36815053 Free PMC article.
-
Engineered biomembrane-derived nanoparticles for nanoscale theranostics.Theranostics. 2023 Jan 1;13(1):20-39. doi: 10.7150/thno.76894. eCollection 2023. Theranostics. 2023. PMID: 36593970 Free PMC article. Review.
-
Tumor Exosome Mimicking Nanoparticles for Tumor Combinatorial Chemo-Photothermal Therapy.Front Bioeng Biotechnol. 2020 Aug 31;8:1010. doi: 10.3389/fbioe.2020.01010. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32984284 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

