BH3-only proteins are dispensable for apoptosis induced by pharmacological inhibition of both MCL-1 and BCL-XL
- PMID: 30185825
- PMCID: PMC6748112
- DOI: 10.1038/s41418-018-0183-7
BH3-only proteins are dispensable for apoptosis induced by pharmacological inhibition of both MCL-1 and BCL-XL
Abstract
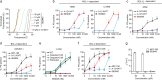
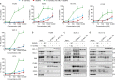
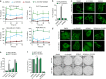
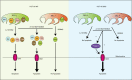
The impressive selectivity and efficacy of BH3 mimetics for treating cancer has largely been limited to BCL-2 dependent hematological malignancies. Most solid tumors depend on other anti-apoptotic proteins, including MCL-1, for survival. The recent description of S63845 as the first specific and potent MCL-1 inhibitor represents an important therapeutic advance, since MCL-1 is not targeted by the currently available BH3 mimetics, Navitoclax or Venetoclax, and is commonly associated with chemoresistance. In this study, we confirm a high binding affinity and selectivity of S63845 to induce apoptosis in MCL-1-dependent cancer cell lines. Furthermore, S63845 synergizes with other BH3 mimetics to induce apoptosis in cell lines derived from both hematological and solid tumors. Although the anti-apoptotic BCL-2 family members in these cell lines interact with a spectrum of pro-apoptotic BH3-only proteins to regulate apoptosis, these interactions alone do not explain the relative sensitivities of these cell lines to BH3 mimetic-induced apoptosis. These findings necessitated further investigation into the requirement of BH3-only proteins in BH3 mimetic-mediated apoptosis. Concurrent inhibition of BCL-XL and MCL-1 by BH3 mimetics in colorectal HCT116 cells induced apoptosis in a BAX- but not BAK-dependent manner. Remarkably this apoptosis was independent of all known BH3-only proteins. Although BH3-only proteins were required for apoptosis induced as a result of BCL-XL inhibition, this requirement was overcome when both BCL-XL and MCL-1 were inhibited, implicating distinct mechanisms by which different anti-apoptotic BCL-2 family members may regulate apoptosis in cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Side-by-side comparison of BH3-mimetics identifies MCL-1 as a key therapeutic target in AML.Cell Death Dis. 2019 Dec 4;10(12):917. doi: 10.1038/s41419-019-2156-2. Cell Death Dis. 2019. PMID: 31801941 Free PMC article.
-
A direct comparison of selective BH3-mimetics reveals BCL-XL, BCL-2 and MCL-1 as promising therapeutic targets in neuroblastoma.Br J Cancer. 2020 May;122(10):1544-1551. doi: 10.1038/s41416-020-0795-9. Epub 2020 Mar 18. Br J Cancer. 2020. PMID: 32203216 Free PMC article.
-
Impact of elevated anti-apoptotic MCL-1 and BCL-2 on the development and treatment of MLL-AF9 AML in mice.Cell Death Differ. 2019 Jul;26(7):1316-1331. doi: 10.1038/s41418-018-0209-1. Epub 2018 Nov 23. Cell Death Differ. 2019. PMID: 30470795 Free PMC article.
-
BH3-Mimetic Drugs: Blazing the Trail for New Cancer Medicines.Cancer Cell. 2018 Dec 10;34(6):879-891. doi: 10.1016/j.ccell.2018.11.004. Cancer Cell. 2018. PMID: 30537511 Review.
-
The first MCL-1-selective BH3 mimetics have therapeutic potential for chronic lymphocytic leukemia.Crit Rev Oncol Hematol. 2016 Apr;100:32-6. doi: 10.1016/j.critrevonc.2016.02.003. Epub 2016 Feb 11. Crit Rev Oncol Hematol. 2016. PMID: 26899021 Review.
Cited by
-
Exploring the potential of BH3 mimetic therapy in squamous cell carcinoma of the head and neck.Cell Death Dis. 2019 Dec 4;10(12):912. doi: 10.1038/s41419-019-2150-8. Cell Death Dis. 2019. PMID: 31801952 Free PMC article.
-
Skp2 stabilizes Mcl-1 and confers radioresistance in colorectal cancer.Cell Death Dis. 2022 Mar 18;13(3):249. doi: 10.1038/s41419-022-04685-0. Cell Death Dis. 2022. PMID: 35301297 Free PMC article.
-
Targeting the Intrinsic Apoptosis Pathway: A Window of Opportunity for Prostate Cancer.Cancers (Basel). 2021 Dec 23;14(1):51. doi: 10.3390/cancers14010051. Cancers (Basel). 2021. PMID: 35008216 Free PMC article. Review.
-
Knockdown of Myeloid Cell Leukemia-1 by MicroRNA-101 Increases Sensitivity of A549 Lung Cancer Cells to Etoposide.Iran J Med Sci. 2021 Jul;46(4):298-307. doi: 10.30476/ijms.2020.83173.1203. Iran J Med Sci. 2021. PMID: 34305242 Free PMC article.
-
Osteosarcoma cells depend on MCL-1 for survival, and osteosarcoma metastases respond to MCL-1 antagonism plus regorafenib in vivo.BMC Cancer. 2024 Nov 4;24(1):1350. doi: 10.1186/s12885-024-13088-7. BMC Cancer. 2024. PMID: 39497108 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

