Mesenchymal Stromal Cell-Seeded Biomimetic Scaffolds as a Factory of Soluble RANKL in Rankl-Deficient Osteopetrosis
- PMID: 30184340
- PMCID: PMC6312453
- DOI: 10.1002/sctm.18-0085
Mesenchymal Stromal Cell-Seeded Biomimetic Scaffolds as a Factory of Soluble RANKL in Rankl-Deficient Osteopetrosis
Abstract
Biomimetic scaffolds are extremely versatile in terms of chemical composition and physical properties, which can be defined to accomplish specific applications. One property that can be added is the production/release of bioactive soluble factors, either directly from the biomaterial, or from cells embedded within the biomaterial. We reasoned that pursuing this strategy would be appropriate to setup a cell-based therapy for RANKL-deficient autosomal recessive osteopetrosis, a very rare skeletal genetic disease in which lack of the essential osteoclastogenic factor RANKL impedes osteoclast formation. The exogenously administered RANKL cytokine is effective in achieving osteoclast formation and function in vitro and in vivo, thus, we produced murine Rankl-/- mesenchymal stromal cells (MSCs) overexpressing human soluble RANKL (hsRL) following lentiviral transduction (LVhsRL). Here, we described a three-dimensional (3D) culture system based on a magnesium-doped hydroxyapatite/collagen I (MgHA/Col) biocompatible scaffold closely reproducing bone physicochemical properties. MgHA/Col-seeded murine MSCs showed improved properties, as compared to two-dimensional (2D) culture, in terms of proliferation and hsRL production, with respect to LVhsRL-transduced cells. When implanted subcutaneously in Rankl-/- mice, these cell constructs were well tolerated, colonized by host cells, and intensely vascularized. Of note, in the bone of Rankl-/- mice that carried scaffolds with either WT or LVhsRL-transduced Rankl-/- MSCs, we specifically observed formation of TRAP+ cells, likely due to sRL released from the scaffolds into circulation. Thus, our strategy proved to have the potential to elicit an effect on the bone; further work is required to maximize these benefits and achieve improvements of the skeletal pathology in the treated Rankl-/- mice. Stem Cells Translational Medicine 2019;8:22-34.
Keywords: Biomimetic scaffold; Cell therapy; Gene therapy; Mesenchymal stromal cell; Osteopetrosis; RANKL.
© 2018 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures



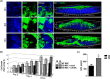
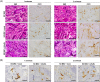
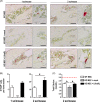
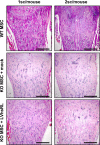
Similar articles
-
Murine Rankl-/- Mesenchymal Stromal Cells Display an Osteogenic Differentiation Defect Improved by a RANKL-Expressing Lentiviral Vector.Stem Cells. 2017 May;35(5):1365-1377. doi: 10.1002/stem.2574. Epub 2017 Mar 1. Stem Cells. 2017. PMID: 28100034
-
Rankl genetic deficiency and functional blockade undermine skeletal stem and progenitor cell differentiation.Stem Cell Res Ther. 2024 Jul 6;15(1):203. doi: 10.1186/s13287-024-03803-3. Stem Cell Res Ther. 2024. PMID: 38971808 Free PMC article.
-
Infantile malignant, autosomal recessive osteopetrosis: the rich and the poor.Calcif Tissue Int. 2009 Jan;84(1):1-12. doi: 10.1007/s00223-008-9196-4. Epub 2008 Dec 12. Calcif Tissue Int. 2009. PMID: 19082854 Review.
-
3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells.Int J Mol Sci. 2018 Oct 13;19(10):3150. doi: 10.3390/ijms19103150. Int J Mol Sci. 2018. PMID: 30322134 Free PMC article. Review.
-
A RANKL G278R mutation causing osteopetrosis identifies a functional amino acid essential for trimer assembly in RANKL and TNF.Hum Mol Genet. 2012 Feb 15;21(4):784-98. doi: 10.1093/hmg/ddr510. Epub 2011 Nov 7. Hum Mol Genet. 2012. PMID: 22068587
Cited by
-
Precision Nanomedicine with Bio-Inspired Nanosystems: Recent Trends and Challenges in Mesenchymal Stem Cells Membrane-Coated Bioengineered Nanocarriers in Targeted Nanotherapeutics.J Xenobiot. 2024 Jun 24;14(3):827-872. doi: 10.3390/jox14030047. J Xenobiot. 2024. PMID: 39051343 Free PMC article. Review.
-
Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice.Stem Cell Res Ther. 2020 Jun 26;11(1):255. doi: 10.1186/s13287-020-01769-6. Stem Cell Res Ther. 2020. PMID: 32586410 Free PMC article.
-
Generation of an immunodeficient mouse model of tcirg1-deficient autosomal recessive osteopetrosis.Bone Rep. 2020 Jan 7;12:100242. doi: 10.1016/j.bonr.2020.100242. eCollection 2020 Jun. Bone Rep. 2020. PMID: 31938717 Free PMC article.
-
Overcoming the Design Challenge in 3D Biomimetic Hybrid Scaffolds for Bone and Osteochondral Regeneration by Factorial Design.Front Bioeng Biotechnol. 2020 Jul 7;8:743. doi: 10.3389/fbioe.2020.00743. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32775321 Free PMC article.
-
Expanded circulating hematopoietic stem/progenitor cells as novel cell source for the treatment of TCIRG1 osteopetrosis.Haematologica. 2021 Jan 1;106(1):74-86. doi: 10.3324/haematol.2019.238261. Haematologica. 2021. PMID: 31949009 Free PMC article.
References
-
- Rebelo MA, Alves TF, de Lima R et al. Scaffolds and tissue regeneration: An overview of the functional properties of selected organic tissues. J Biomed Mater Res B Appl Biomater 2016;104(7):1483–1494. - PubMed
-
- Li, J.J. , Ebied M., Xu J., and Zreiqat H., Current Approaches to Bone Tissue Engineering: The Interface between Biology and Engineering. Adv Healthc Mater, 2017. - PubMed
-
- Fernandez‐Yague MA, Abbah SA, McNamara L et al. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv Drug Deliv Rev 2015;84:1–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

