Contribution of Adrenal Glands to Intratumor Androgens and Growth of Castration-Resistant Prostate Cancer
- PMID: 30181386
- PMCID: PMC6320302
- DOI: 10.1158/1078-0432.CCR-18-1431
Contribution of Adrenal Glands to Intratumor Androgens and Growth of Castration-Resistant Prostate Cancer
Abstract
Purpose: Tumor androgens in castration-resistant prostate cancer (CRPC) reflect de novo intratumoral synthesis or adrenal androgens. We used C.B.-17 SCID mice in which we observed adrenal CYP17A activity to isolate the impact of adrenal steroids on CRPC tumors in vivo.
Experimental design: We evaluated tumor growth and androgens in LuCaP35CR and LuCaP96CR xenografts in response to adrenalectomy (ADX). We assessed protein expression of key steroidogenic enzymes in 185 CRPC metastases from 42 patients.
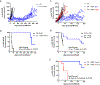
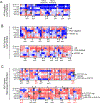
Results: Adrenal glands of intact and castrated mice expressed CYP17A. Serum DHEA, androstenedione (AED), and testosterone (T) in castrated mice became undetectable after ADX (all P < 0.05). ADX prolonged median survival (days) in both CRPC models (33 vs. 179; 25 vs. 301) and suppressed tumor steroids versus castration alone (T 0.64 pg/mg vs. 0.03 pg/mg; DHT 2.3 pg/mg vs. 0.23 pg/mg; and T 0.81 pg/mg vs. 0.03 pg/mg, DHT 1.3 pg/mg vs. 0.04 pg/mg; all P ≤ 0.001). A subset of tumors recurred with increased steroid levels, and/or induction of androgen receptor (AR), truncated AR variants, and glucocorticoid receptor (GR). Metastases from 19 of 35 patients with AR positive tumors concurrently expressed enzymes for adrenal androgen utilization and nine expressed enzymes for de novo steroidogenesis (HSD3B1, CYP17A, AKR1C3, and HSD17B3).
Conclusions: Mice are appropriate for evaluating adrenal impact of steroidogenesis inhibitors. A subset of ADX-resistant CRPC tumors demonstrate de novo androgen synthesis. Tumor growth and androgens were suppressed more strongly by surgical ADX than prior studies using abiraterone, suggesting reduction in adrenally-derived androgens beyond that achieved by abiraterone may have clinical benefit. Proof-of-concept studies with agents capable of achieving true "nonsurgical ADX" are warranted.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors declare no financial conflicts of interest.
Figures



Similar articles
-
Abiraterone switches castration-resistant prostate cancer dependency from adrenal androgens towards androgen receptor variants and glucocorticoid receptor signalling.Prostate. 2022 Apr;82(5):505-516. doi: 10.1002/pros.24297. Epub 2022 Jan 17. Prostate. 2022. PMID: 35037287 Free PMC article.
-
Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer.Cancer Res. 2010 Feb 1;70(3):1256-64. doi: 10.1158/0008-5472.CAN-09-2092. Epub 2010 Jan 19. Cancer Res. 2010. PMID: 20086173
-
Nuclear Receptor LRH-1 Functions to Promote Castration-Resistant Growth of Prostate Cancer via Its Promotion of Intratumoral Androgen Biosynthesis.Cancer Res. 2018 May 1;78(9):2205-2218. doi: 10.1158/0008-5472.CAN-17-2341. Epub 2018 Feb 8. Cancer Res. 2018. PMID: 29438990
-
Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis.Oncogene. 2014 May 29;33(22):2815-25. doi: 10.1038/onc.2013.235. Epub 2013 Jun 10. Oncogene. 2014. PMID: 23752196 Free PMC article. Review.
-
HSD3B1 Genotypes Conferring Adrenal-Restrictive and Adrenal-Permissive Phenotypes in Prostate Cancer and Beyond.Endocrinology. 2019 Sep 1;160(9):2180-2188. doi: 10.1210/en.2019-00366. Endocrinology. 2019. PMID: 31271415 Free PMC article. Review.
Cited by
-
Role of androgen receptor splice variant-7 (AR-V7) in prostate cancer resistance to 2nd-generation androgen receptor signaling inhibitors.Oncogene. 2020 Nov;39(45):6935-6949. doi: 10.1038/s41388-020-01479-6. Epub 2020 Sep 28. Oncogene. 2020. PMID: 32989253 Free PMC article.
-
Anti-Müllerian hormone: a novel biomarker for aggressive prostate cancer? Emerging evidence from a prospective study of radical prostatectomies.Hormones (Athens). 2024 Jun;23(2):297-304. doi: 10.1007/s42000-023-00520-z. Epub 2023 Dec 21. Hormones (Athens). 2024. PMID: 38127275 Free PMC article.
-
Differential but Concerted Expression of HSD17B2, HSD17B3, SHBG and SRD5A1 Testosterone Tetrad Modulate Therapy Response and Susceptibility to Disease Relapse in Patients with Prostate Cancer.Cancers (Basel). 2021 Jul 12;13(14):3478. doi: 10.3390/cancers13143478. Cancers (Basel). 2021. PMID: 34298692 Free PMC article.
-
Targeting sex steroid biosynthesis for breast and prostate cancer therapy.Nat Rev Cancer. 2023 Sep 8. doi: 10.1038/s41568-023-00609-y. Online ahead of print. Nat Rev Cancer. 2023. PMID: 37684402 Review. No abstract available.
-
Testosterone accumulation in prostate cancer cells is enhanced by facilitated diffusion.Prostate. 2019 Sep;79(13):1530-1542. doi: 10.1002/pros.23874. Epub 2019 Aug 2. Prostate. 2019. PMID: 31376206 Free PMC article.
References
-
- Scher HI. Current management of hormone-refractory prostate cancer. Clin Adv Hematol Oncol 2004;2(11):724–6. - PubMed
-
- Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 2006;66(5):2815–25. - PubMed
-
- Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 2014;32(33):3705–15. - PMC - PubMed
-
- Arai S, Miyashiro Y, Shibata Y, Tomaru Y, Kobayashi M, Honma S, et al. Effect of castration monotherapy on the levels of adrenal androgens in cancerous prostatic tissues. Steroids. 2011;76(3):301–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

