Exploring the mechanisms behind long noncoding RNAs and cancer
- PMID: 30175284
- PMCID: PMC6114262
- DOI: 10.1016/j.ncrna.2018.03.001
Exploring the mechanisms behind long noncoding RNAs and cancer
Abstract
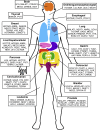
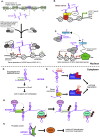
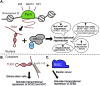
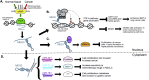
Over the past decade, long noncoding RNAs (lncRNAs) have been identified as significant players in gene regulation. They are often differentially expressed and widely-associated with a majority of cancer types. The aberrant expression of these transcripts has been linked to tumorigenesis, metastasis, cancer stage progression and patient survival. Despite their apparent link to cancer, it has been challenging to gain a mechanistic understanding of how they contribute to cancer, partially due the difficulty in discriminating functional RNAs from other noncoding transcription events. However, there are several well-studied lncRNAs where specific mechanisms have been more clearly defined, leading to new discoveries into how these RNAs function. One major observation that has come to light is the context-dependence of lncRNA mechanisms, where they often have unique function in specific cell types and environment. Here, we review the molecular mechanisms of lncRNAs with a focus on cancer pathways, illustrating a few informative examples. Together, this type of detailed insight will lead to a greater understanding of the potential for the application of lncRNAs as targets of cancer therapies and diagnostics.
Keywords: Cancer; Chromatin; HOTAIR; MEG3; TUG1; lncRNA.
Figures


Similar articles
-
Long noncoding RNA in genome regulation: prospects and mechanisms.RNA Biol. 2010 Sep-Oct;7(5):582-5. doi: 10.4161/rna.7.5.13216. Epub 2010 Sep 1. RNA Biol. 2010. PMID: 20930520 Free PMC article.
-
Long noncoding RNAs: Novel insights into hepatocelluar carcinoma.Cancer Lett. 2014 Mar 1;344(1):20-27. doi: 10.1016/j.canlet.2013.10.021. Epub 2013 Oct 30. Cancer Lett. 2014. PMID: 24183851 Review.
-
Long non-coding RNAs: From disease code to drug role.Acta Pharm Sin B. 2021 Feb;11(2):340-354. doi: 10.1016/j.apsb.2020.10.001. Epub 2020 Oct 10. Acta Pharm Sin B. 2021. PMID: 33643816 Free PMC article. Review.
-
Long noncoding RNAs in the initiation, progression, and metastasis of hepatocellular carcinoma.Noncoding RNA Res. 2017 Dec 5;2(3-4):129-136. doi: 10.1016/j.ncrna.2017.11.001. eCollection 2017 Sep. Noncoding RNA Res. 2017. PMID: 30159431 Free PMC article. Review.
-
Long noncoding RNA in prostate, bladder, and kidney cancer.Eur Urol. 2014 Jun;65(6):1140-51. doi: 10.1016/j.eururo.2013.12.003. Epub 2013 Dec 14. Eur Urol. 2014. PMID: 24373479 Review.
Cited by
-
LncRNA-536 and RNA Binding Protein RBM25 Interactions in Pulmonary Arterial Hypertension.bioRxiv [Preprint]. 2024 Aug 28:2024.08.27.610011. doi: 10.1101/2024.08.27.610011. bioRxiv. 2024. PMID: 39253448 Free PMC article. Preprint.
-
Exploring potential roles of long non-coding RNAs in cancer immunotherapy: a comprehensive review.Front Immunol. 2024 Aug 27;15:1446937. doi: 10.3389/fimmu.2024.1446937. eCollection 2024. Front Immunol. 2024. PMID: 39257589 Free PMC article. Review.
-
Long noncoding RNAs: glycolysis regulators in gynaecologic cancers.Cancer Cell Int. 2023 Jan 13;23(1):4. doi: 10.1186/s12935-023-02849-2. Cancer Cell Int. 2023. PMID: 36639695 Free PMC article. Review.
-
Long Non-coding RNAs Involved in Resistance to Chemotherapy in Ovarian Cancer.Front Oncol. 2020 Jan 21;9:1549. doi: 10.3389/fonc.2019.01549. eCollection 2019. Front Oncol. 2020. PMID: 32039022 Free PMC article. Review.
-
Regulation of RUNX proteins by long non-coding RNAs and circular RNAs in different cancers.Noncoding RNA Res. 2021 May 30;6(2):100-106. doi: 10.1016/j.ncrna.2021.05.001. eCollection 2021 Jun. Noncoding RNA Res. 2021. PMID: 34189363 Free PMC article. Review.
References
-
- Rutenberg-Schoenberg M., Sexton A.N., Simon M.D. The properties of long noncoding RNAs that regulate chromatin. Annu. Rev. Genom. Hum. Genet. 2016;17:69–94. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

