Yap/Taz mediates mTORC2-stimulated fibroblast activation and kidney fibrosis
- PMID: 30154246
- PMCID: PMC6200934
- DOI: 10.1074/jbc.RA118.004073
Yap/Taz mediates mTORC2-stimulated fibroblast activation and kidney fibrosis
Abstract
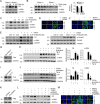
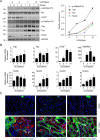
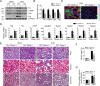
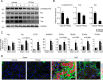
Our previously published study demonstrated that mammalian target of rapamycin complex 2 (mTORC2) signaling mediates TGFβ1-induced fibroblast activation. However, the underlying mechanisms for mTORC2 in stimulating fibroblast activation remain poorly understood. Here, we found that TGFβ1 could stimulate mTORC2 and Yap/Taz activation in NRK-49F cells. Blocking either mTORC2 or Yap/Taz signaling diminished TGFβ1-induced fibroblast activation. In addition, blockade of mTORC2 could down-regulate the expression of Yap/Taz, connective tissue growth factor (CTGF), and ankyrin repeat domain 1 (ANKRD1). Overexpression of constitutively active Taz (Taz-S89A) could restore fibroblast activation suppressed by PP242, an mTOR kinase inhibitor in NRK-49F cells. In mouse kidneys with unilateral ureter obstructive (UUO) nephropathy, both mTORC2 and Yap/Taz were activated in the interstitial myofibroblasts. Ablation of Rictor in fibroblasts/pericytes or blockade of mTOR signaling with PP242 attenuated Yap/Taz activation and UUO nephropathy in mice. Together, this study uncovers that targeting mTORC2 retards fibroblast activation and kidney fibrosis through suppressing Yap/Taz activation.
Keywords: Rictor; Yes-associated protein (YAP); cell signaling; fibroblast; fibrosis; kidney.
© 2018 Gui et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Rictor/mTORC2 signaling mediates TGFβ1-induced fibroblast activation and kidney fibrosis.Kidney Int. 2015 Sep;88(3):515-27. doi: 10.1038/ki.2015.119. Epub 2015 May 13. Kidney Int. 2015. PMID: 25970154 Free PMC article.
-
Yap/Taz Deletion in Gli+ Cell-Derived Myofibroblasts Attenuates Fibrosis.J Am Soc Nephrol. 2017 Nov;28(11):3278-3290. doi: 10.1681/ASN.2015121354. Epub 2017 Aug 2. J Am Soc Nephrol. 2017. PMID: 28768710 Free PMC article.
-
The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz.J Biol Chem. 2018 Dec 14;293(50):19290-19302. doi: 10.1074/jbc.RA118.005457. Epub 2018 Oct 17. J Biol Chem. 2018. PMID: 30333225 Free PMC article.
-
Insights into the Regulation of Yap/Taz from Cellular Systems and Mouse Models.Curr Stem Cell Res Ther. 2018;13(1):16-25. doi: 10.2174/1574888X12666170102124421. Curr Stem Cell Res Ther. 2018. PMID: 28042766 Review.
-
YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer.Int J Mol Sci. 2018 Nov 20;19(11):3674. doi: 10.3390/ijms19113674. Int J Mol Sci. 2018. PMID: 30463366 Free PMC article. Review.
Cited by
-
Mammalian Target of Rapamycin Complex 2 Signaling Is Required for Liver Regeneration in a Cholestatic Liver Injury Murine Model.Am J Pathol. 2020 Jul;190(7):1414-1426. doi: 10.1016/j.ajpath.2020.03.010. Epub 2020 Apr 7. Am J Pathol. 2020. PMID: 32275903 Free PMC article.
-
Loss of Rictor in tubular cells exaggerates lipopolysaccharide induced renal inflammation and acute kidney injury via Yap/Taz-NF-κB axis.Cell Death Discov. 2020 May 29;6:40. doi: 10.1038/s41420-020-0274-3. eCollection 2020. Cell Death Discov. 2020. PMID: 32528729 Free PMC article.
-
The mTORC2 signaling network: targets and cross-talks.Biochem J. 2024 Jan 25;481(2):45-91. doi: 10.1042/BCJ20220325. Biochem J. 2024. PMID: 38270460 Free PMC article. Review.
-
Verteporfin ameliorates fibrotic aspects of Dupuytren's disease nodular fibroblasts irrespective the activation state of the cells.Sci Rep. 2022 Aug 17;12(1):13940. doi: 10.1038/s41598-022-18116-9. Sci Rep. 2022. PMID: 35977978 Free PMC article.
-
Novel zebrafish polycystic kidney disease models reveal functions of the Hippo pathway in renal cystogenesis.Dis Model Mech. 2021 Nov 1;14(11):dmm049027. doi: 10.1242/dmm.049027. Epub 2021 Nov 9. Dis Model Mech. 2021. PMID: 34545930 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

